Damage caused by acid rain has been well-documented leading to it being labelled as an environmental hazard. So, how does acid rain affect the environment? Here we examine how it alters Earth’s natural processes, and consider the types of damage to the living world that can occur as a result of these changes.
—
Acid rain can be defined as precipitation that is abnormally acidic due to it containing dissolved pollutants, which make it capable of causing great environmental harm. Typical rain will have a pH of around 5.5 whereas the pH of acid rain is much lower at around 4.0 due to it containing dissolved sulphur dioxide or nitrogen oxides, which are acidic pollutants.
How Does Acid Rain Affect the Atmosphere
The majority of the emissions of sulphur dioxide and nitrogen oxides come from human activities such as burning of fossil fuels or vehicle exhaust fumes. However, a small fraction of emissions exist from natural processes such as decaying vegetation and volcanic activity.
These emissions of sulphur dioxide and nitrogen oxide diffuse into the atmosphere and dissolve in water droplets in clouds forming sulphuric acid and nitric acid respectfully. Clouds containing these acidic droplets can then be transported by winds before precipitation occurs, creating acid rain through a process known as wet deposition. Alternatively, some of the pollutant particles may not become dissolved in cloud water to form acid rain so instead return to Earth’s surface through dry deposition.
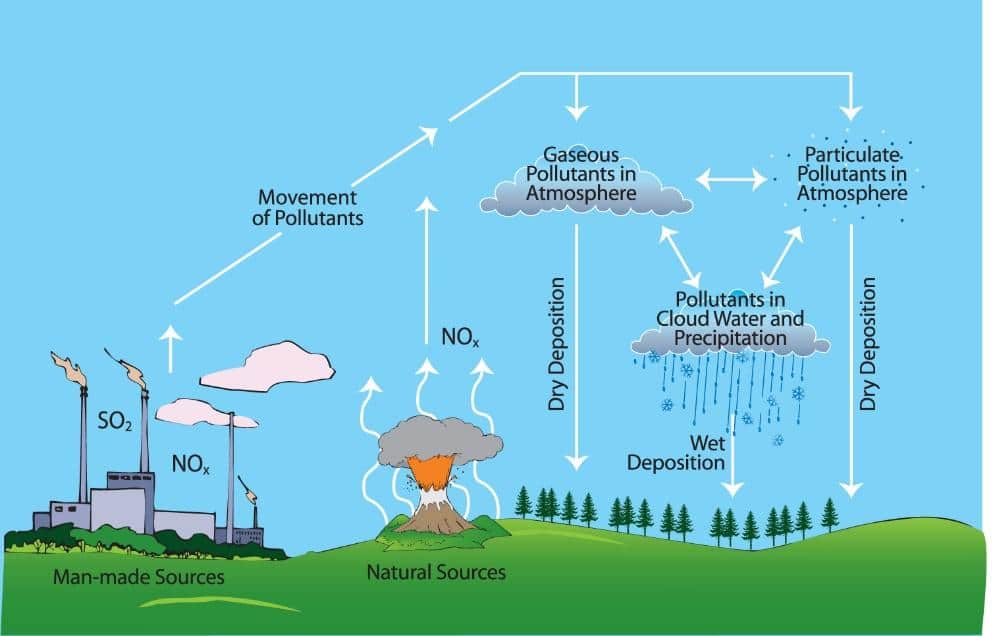
How does acid rain form? Image: US Environmental Protection Agency.
How Does Acid Rain Affect the Water Cycle?
After being released from clouds as precipitation, acid rain reaches the Earth’s surface and a large fraction of it is transported to rivers and lakes through surface runoff or by groundwater flow. Here, it mixes with the existing water and increases the acidity of the water body with this drop in pH being particularly dramatic when large volumes of rainfall enter a relatively small water body.
In addition to rainfall, acid rain can also be deposited from the atmosphere as acid snow when temperatures are cold enough. This form of acid deposition can be particularly devastating to the natural environment as it will accumulate on the ground before suddenly melting to release a large volume of acidic water into the surrounding landscape.
If you enjoyed learning how does acid rain affect the environment, you might also like: What are the Biggest Causes and Effects of Air Pollution?
How Does Acid Rain Affect Soil and Rock?
Land surfaces that are made up of limestone rock are vulnerable to erosion from acid rain as the calcium carbonate in limestone reacts to the acidity, producing calcium sulphate or calcium nitrate which are both soluble products. The reaction releases carbon dioxide gases as well. Water will eventually transport the soluble products into river systems where the concentration of it may be high enough to cause damage to aquatic life. Additionally, carbon dioxide released from the reaction will enter the atmosphere where it will contribute to and exacerbate global warming.
The decrease in pH caused by the acid rain also impacts the concentration of different heavy metals that are present in the surrounding water. For example, a more acidic environment allows aluminium to be more readily released from the soil into the surrounding water whereas calcium becomes less readily available, so its concentration in the water is lower. The increase in concentration of some heavy metals in the water may make it toxic to sensitive aquatic organisms and equally, the reduction of some metals that may be crucial to an organism’s survival could have damaging effects on the ecosystem.

Example of a standard pH mediated, nutrient availability chart for aquatically cultured plants. Red line represents a normal operating pH for a hydroponic system; the blue line that for an aquaponic system. Source: Simon Goddek/ResearchGate.
How Does Acid Rain Affect Plant Growth and Ecosystems
Living organisms suffer directly from acid rain falling in their habitat with species living in confined aquatic environments being particularly vulnerable as they cannot migrate to less acidic waters. Whilst some species have a high tolerance to acidic conditions, others cannot survive even very small changes in pH. For example, the increased acidity in several lochs in Galloway, Scotland in the 1900s led to the local extinction of several of the local fish populations.
The waxy outer layer of plant leaves can also become damaged by acid rain and the inability to photosynthesise efficiently makes the plant weak with an increased chance of mortality. The initial loss of key species in an ecosystem due to their high sensitivity to acid rain can result in the subsequent loss of further species who were dependent on the key species for their own survival, and this may result in the collapse of entire ecosystems.
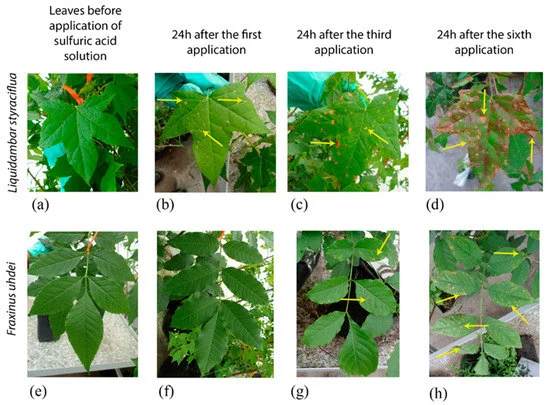
Simulation of leaf damage that can be caused by acid rain. Image: Verónica et al. (2020)
How Does Acid Rain Affect Human Health?
Acid rain and the pollutant particles of sulphur dioxide and nitrogen oxide that it is formed from have been linked to human health problems including asthma, heart disease and eye irritation. In addition to forming acid rain, nitrogen oxides are also known to be involved in a reaction which creates tropospheric ozone which is known to cause respiratory problems in humans.
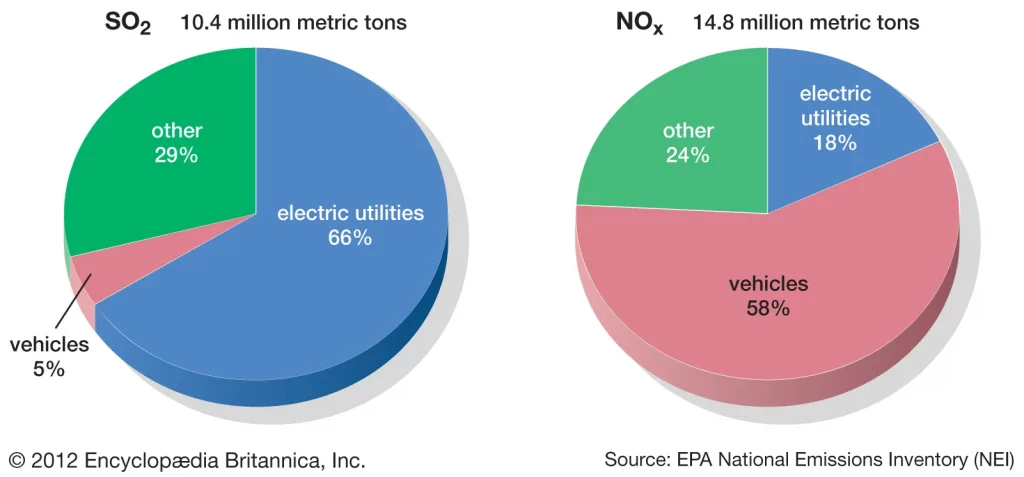
In answering the question on how does acid rain affect the environment, one will discover a whole host of environmental problems and impacts on humans. To prevent further damage from acid rain, it is important that we identify the main sources of sulphur dioxide and nitrogen oxide pollution and cut these emissions to meet higher air quality standards. Cutting emissions from these polluting sectors such as electric utilities and vehicles requires cleaner technologies to be used which can scrub out the pollutant gases and prevent them from causing environmental damage.
Featured image by: Flickr (CC BY-NC-ND 2.0)
You might also like: Air Pollution: Have We Reached the Point of No Return?