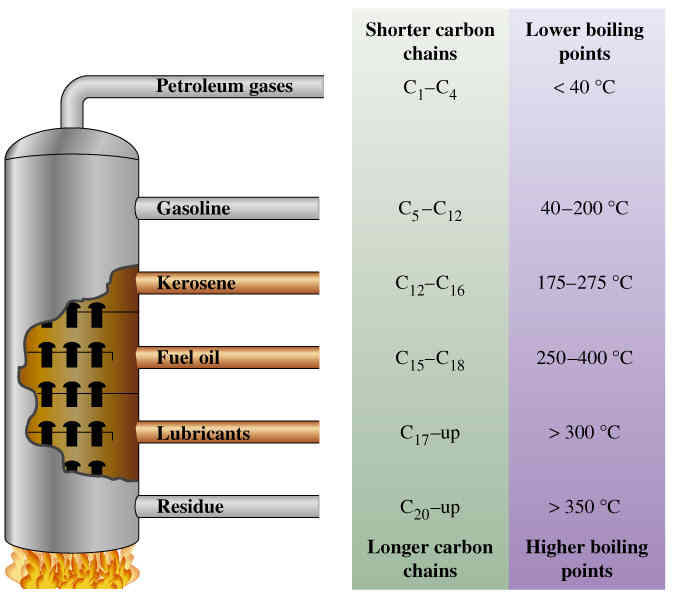
Fractional distillation is the process of separating a substance into its parts (or fractions), taking advantage of different vapor pressure properties of those substances. Fractional distillation is often used as a synonym with “distillation” because distillation always takes advantage of a difference in boiling points of component substances for separation.

- Add heat to a liquid mixture with two or more main substances; for example, a water and ethanol mixture.
- As the liquid heats, components with the lower boiling points will begin to vaporize and rise through the column. In the water/ethanol example, ethanol will boil off first (BP 78° C, compared to water (BP 100° C). However, the vapor rising will still contain some molecules of the other substances. Vapor gets purer the higher it rises in the column, as heavier molecules “fall off” and turn back to liquid.
- As vapor rises in the distillation column, heavier molecules will condense back into liquid and “rain” back down. At any given point in a fractionating column, vapor will be rising, liquid will be falling, and molecules will be mixing. Columns naturally have certain “stages”; a stage is an area in the column with a similar amount of molecules of each type of substance (i.e. a general certain percentage of water and ethanol). Columns are designed to be tall enough to achieve a certain percentage separation, by finding the minimum number of required stages.
- Vapor reaching the top of the column (distillate) is collected into an industrial condenser (a big chiller), which cools the vapor back into a liquid, and piped to tank or storage.
- Substances remaining in the column continue the process of distillation, until the desired purity is reached. Some columns are a continuous process (most common), where new base solution is added continually. Others are batch systems, where the base is removed when a desired separation is achieved. In many systems, solution is recirculated several times to make sure substances are properly separated.
What is described above is the basic process of distillation. A distillation column is sometimes referred to as a fractional distillation column in industry. Columns that only separate two substances can be called fractionating columns. A fractional distillation system usually achieves several different products at multiple points within the column. As substances rise, mixtures can be pulled at various stages, and condensed.
Do you need an industrial distillation system? Contact | 314-806-1678 for one of our distillation experts, or find out more about distillation column design today.
Related Links:
- 91% ethanol removal – A distillation system with an innovative design by EPIC extracted 91% of the ethanol in a water/ethanol mixture. Read the distillation system case study…
- Basic Distillation Column Design – What are the basic components to consider when designing a distillation column? Check out this distillation column design infographic to learn more.
- Steps to acquire a custom distillation system – Looking for a custom distillation system? Your first step is to connect with a distillation system design and build firm, and explore your distillation goals. Learn more about distillation system design...