Abstract
Low-alcohol beer (LAB) is a growing part of the brewing industry in terms of market volumes and consumer interest. Universities and research centres are making efforts to improve organoleptic profile and flavour stability of the product. One of the main limitations of such products is the stability. These beers must be severely filtered and pasteurized, causing a significant loss of quality in terms of flavour. Herein, flavour stability of an unpasteurized and unfiltered LAB was checked during 120 days of cold storage (4 ± 1 °C). The results showed that the beer remained stable for 120 days for many observed parameters. The alcohol content increased from 0.5 to 0.7% v/v. The beer without oxygen was more stable than that filled with oxygen in the headspace. The results confirmed the possibility to produce an unpasteurized craft LAB by Saccharomycodes ludwigii by the cold chain.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low-alcohol beer (LAB) is one of the biggest future challenges for the brewing industry, witnessed by the increasing volume produced. Consumers are more and more interested in it, because of the promotion of a healthier way of life and the raise of no-alcohol statements and policies (Marques et al., 2022). However, there is not a worldwide unique definition of low-alcohol and alcohol-free beer. In fact, the European Union (EU) has no regulation that refers to “low-alcohol” and “alcohol-free” (AFB) beers; consequently, each country has its own legislation (Montanari et al., 2008). Most of the European regulations define LAB and AFB with different specifications. For example, for AFB, ethanol content should be below 1% v/v in Spain, no more than 0.5% v/v in Austria, Belgium, Finland, Germany and Portugal and below 0.1% in Netherland. The lowest alcohol limit for AFB is in the UK, with a value of ≤ 0.05% v/v (De Francesco et al., 2018). The alcohol content of LAB should be below 1.2% in Austria, Belgium, Finland, Germany, Italy, Netherlands, Portugal and the UK. Finland, Spain and Sweden represent an exception; in fact, LABs have respectively < 2.8%, 1 to 3% and ≤ 2.25% v/v of alcohol (Montanari et al., 2008). Among the North American countries, the US regulation states that LAB should have a maximum alcohol content of 2.5%, while for AFB the ethanol content must be below 0.5% v/v. According to Food and Drug Regulation, in Canada, a beer with ethanol content lower than 1.1% v/v can be classified as LAB. The growing commercial demand for LAB and AFB due to the increasing of a healthier lifestyle, the ethical consumption in different world areas and the rising of craft breweries have recently stimulated researches focused on obtaining low ethanol beverages with improved stability and with a better taste (Bellut & Arendt, 2019). The global beer market is growing at different rates. According to Bellut and Arendt (2019), the worldwide AFB and LAB total volumes increased respectively by 21 and 20% in the 5-year period 2011–2016, by another 24% in the 5-year period 2011–2016 and are forecast to grow the next years. In Italy, in 2019, LAB and AFB markets stated at 1.60% of the total Italian market, according to the Assobirra report.
Recently, also craft breweries are producing LAB and AFB. Some of them are focused on AFB production, such as Nirvana Brewery (London, UK), Big Drop Brewery (Ipswich, UK) and Infinite Session (London, UK). Anyway, there is no information about how those breweries are making LAB and AFB.
According to De Francesco et al. (2018), beers with reduced alcohol concentration can be produced using physical and biological processes. Physical methods removed alcohol from regular beer using thermal or membrane-mediated processes. They have the great advantage to remove ethanol from beers to very low levels (De Francesco et al., 2014, 2015a).
Among physical methods, osmotic distillation (De Francesco et al., 2014; Liguori et al., 2018; Petrucci et al., 2021), evaporative pertraction (Liguori et al., 2015), pervaporation (Halama et al., 2019), dialysis, vacuum distillation, reverse osmosis, vacuum rectification and evaporation, or spinning cone column distillation are widely studied. As reported in earlier studies, when the processes are well optimized, the dealcoholized beers are usually acceptable in organoleptic and sensory properties. Nevertheless, physical methods require investments beyond the possibilities of craft breweries. Consequently, biological processes are the easiest way to produce a LAB for craft breweries. Interrupted fermentation and non-Saccharomyces strains are the main used biological methods to produce LAB. Interrupted fermentation by Saccharomyces cerevisiae or pastorianus strains is a well-known way to produce a reduced alcohol content beer (Bellut et al., 2018; Liguori et al., 2018; Montanari et al., 2008). On the other hand, non-Saccharomyces yeasts unable to metabolize maltose can be used to obtain LAB; thus, they produce a low amount of ethanol during fermentation of brewing wort. Some papers already published described in detail the use of strains such as Saccharomycodes ludwigii (Callejo et al., 2019; De Francesco et al., 2015a, b; Jackowski & Trusek, 2018), Mrakia spp. (De Francesco et al., 2018) and Pichia spp. (Varela & Varela, 2019). All LABs or AFBs on the market are different from regular beer; anyway many efforts are performed to produce it as enjoyable as possible (Brendel et al., 2020). This is due to the weaker body, poorer flavour and also to pasteurization treatment (Peña-Gómez et al., 2020). Pasteurization increases the level of oxidation in beer, which results in the loss of antioxidants rising the formation of off-flavour, in particular the aldehydes (Lund et al., 2012; Shen et al., 2014). Recently, a review highlighted the possible strategies to remove aldehydes from LABs and AFBs (Gernat et al., 2020). Further, LABs and AFBs, due to the high residual sugars, must be more heavily pasteurized than regular beer (Feilner & Oehmichen, 2016). For this, non-thermal stabilization treatments have been studied to prolong LAB and AFB shelf life, such as high-pressure processing (Milani et al., 2016), ultraviolet irradiation or ultrasound (Nunes et al., 2022). However, they have some implementation issues, such as limited efficacy, changes in beer quality lowering consumer preference and high investment and production costs. Over the microbiological stability, LABs are susceptible to the presence of oxygen. It is well known that ethanol, higher alcohols and esters have a masking effect on beer off-flavours (Tomasi et al., 2017). LABs and AFBs are poor in these volatile compounds; thus, oxidation could easily alter the quality of these kinds of beer. Oxygen content is a factor that can affect LAB and AFB shelf life. Oxygen can be reduced by lowering the intake by the adoption of a modern filling plant that ensures an oxygen uptake below 100 ppb of oxygen. Unfortunately, many small craft breweries do not have such plants because of the excessive costs. LABs have usually been severely pasteurized to guarantee microbiological stability and alcohol content that fits the law limit. However, in some countries, microfiltration and pasteurization are forbidden by law to produce a craft beer (Italian beer law, n.1354/62). To our knowledge, there are no works about the flavour stability of an unpasteurized LAB. Liguori et al. (2016) studied flavour profile of an unpasteurized LAB beer, but in that case was obtained by osmotic distillation and beers were not monitored during storage. In this work, the stability of an unfiltered and unpasteurized LAB bottled with a low and a high amount of oxygen was observed for 120 days of cold storage. The main aim was to check the feasibility to produce an unpasteurized LAB and to evaluate the opportunity to enrich the product portfolio of a craft microbrewery.
Materials and Methods
Yeast Management
Wort was cooled at 25 °C and inoculated with liquid propagated yeast Saccharomycodes ludwigii TUM SL 17 (Hefebank TUM, München, Germany). The propagation of Saccharomycodes ludwigii was conducted with 12°P standard wort diluted to 6°P. Considering that Saccharomycodes ludwigii is not able to ferment maltose, 30 g/l of monohydrate dextrose and 20 g/l of yeast nutrient (FERMIREG ST, Enolife Srl, Taranto, Italy) were added. Then, 10 ml of wort was transferred to a 10-ml plastic flask. The sterilized wort was inoculated with the yeast. After 24 h, the inoculated wort was added to 600 ml of sterilized wort, in aerated conditions at 25 °C. After 48 h, 7.8 * 107 CFU/ml were counted. Then, 600 ml of wort with Saccharomycodes ludwigii was added to 20 l of experimental wort. Finally, after 72 h of aeration at 25 °C, 20 l of wort was added to the whole batch produced.
Brewing Procedure
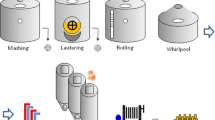
LAB prototypes tested were produced using the 110-l pilot plant at CERB (Italian Brewing Research Centre, Perugia, Italy). The wort was produced by a multiple-step infusion mashing. Pilsner malt (95%; Mouterij Dingemans, Stabroek, Belgium) and CaraRed malt (5%; Weyermann, Bamberg, Germany) were crushed in a 120 kg/h two-roller mill (ENGL Maschinen Gmbh, Schwebheim, Germany) with a 0.5-mm gap between the rollers. After that, the grist was mixed with water in a ratio of 1:2.8 (w/w) to start the mashing process. The mashing started at 75 °C for 25 min, then, after complete saccharification, the temperature raised to 78 °C for 5 min (mash-out). The wort was produced using a temperature higher than β-amylases optimum to get as high dextrin as possible instead of maltose. Perle hop (BarthHaas, Nurnberg, Germany) (10 International Bitterness Units, IBU) was added at the beginning of the boiling. Boiling was 60 min long, followed by a clarification into the whirlpool vessel. Sterile air was injected for 5 min at the bottom of the fermenter through a stainless-steel porous candle before the yeast pitching. The fermentation (15 days at 16 °C) was stopped by a cold crash when the wort density was stable. Maturation took place for 10 days at 1 °C. Beer samples were bottled using a CO2 regulator and 33-cl standard glass dark bottles. For each batch, half of the samples were bottled in isobaric conditions pre-evacuating empty bottles with CO2 before filling and then fobbing after filling (sample A). The other half of the samples were bottled without pre-evacuating and fobbing (sample B). All the samples were capped with crown cap manually and they were put in closed boxes stored at 4 ± 1 °C for the monitoring time. All experimental trials were produced in duplicate.
Wort Analysis
The following analyses were performed at CERB laboratories in duplicate, according to the standard Analytica-European Brewery Convention (EBC) methods. Extract of Wort, EBC method 8.3; pH of Wort, EBC method 8.17; Colour of Wort: Spectrophotometric Method, EBC method 8.5; Fermentability, Attenuation Limit of Wort Reference Fermentation, EBC method 8.6.1 (Analytica-EBC, 2007) were performed.
Beer Analysis
Beer analyses were carried out at CERB laboratories in duplicate by standard Analytica-EBC and MEBAK procedures. Alcohol in Beer by Enzymatic Method, EBC method 9.3; Original, Real and Apparent Extract of Beer, EBC method 9.4; Apparent Degree of Fermentation (ADF), MEBAK method 2.8.4; pH of Beer, EBC method 9.35; Colour of Beer: Spectrophotometric method, EBC method 9.6; Haze in Beer by haze meters, EBC method 9.29; Foam Stability of Beer by NIBEM-T meter, EBC method 9.42.1 (Analytica-EBC, 2007; MEBAK, 2013).
Carbon dioxide (CO2), dissolved oxygen (DO) and total package oxygen (TPO) content were measured with Portable Optical CO2/O2/TPO Meter C-Dgm (Pentair Haffmans, Venlo, Netherlands). DO was measured without sample manipulation while TPO was measured after 5 min of thermostatic agitation and 5 min of rest. Samples and oximeter were conditioned with CO2 (2 bar) and with a standard refrigerated beer.
Sugar Profile
Sugar concentration (g/l) determination in worts and beers was performed in duplicate by high-performance liquid chromatography (HPLC) coupled with evaporative light scattering detector (ELSD) as previously described (Floridi et al., 2001).
Volatile Compounds
Volatile compounds were identified according to the method of De Francesco et al. (2015a, b), which is based on solid-phase microextraction with on-fibre derivatization. Two different analytical methods were used, one for the analysis of vicinal diketones and aldehydes and another for the analysis of higher alcohols and esters. A gas chromatograph (Agilent Technologies model 6850) equipped with a mass spectrometer (Agilent Technologies model 5975C) coupled with a Gerstel Multi-Purpose Sampler (Maestro Autosamples, Baltimore, MD, USA). The gas chromatograph–mass spectrometer was equipped with a glass direct inlet liner (1.5 mm inner diameter and 140 μl volume) and a DB-5MS capillary column of 60 m × 0.32 mm × 1 μm (J&W Scientific, Folsom, CA, USA) consisting of cross-linked 5% phenyl methyl siloxane. A 65-μm poly(dimethyl siloxane)/divinyl benzene (PDMS/DVB) fibre coating (Supelco, Bellefonte, PA, USA) was used. Data analysis was performed using the MSD ChemStation Data Analysis Software (Agilent). The analysed compounds were acetaldehyde, 2-methylbutanal, 3-methylbutanal, hexanal, trans-2 nonenal, phenylacetaldehyde, methional and furfural for aldehydes; diacetyl and 2.3 pentandione for vicinal diketones; n-propanol, isobutanol, 3-methyl-1-butanol, 2-methyl-1-butanol, 2-phenylethan-1-ol and furfuryl alcohol for alcohol and ethyl acetate, ethyl butanoate, isoamyl acetate, ethyl hexanoate and ethyl octanoate for esters.
Sensory Evaluation
The sensory evaluation was performed by a trained panel (composed of 12 people aged between 24 and 50 years) through description analysis according to Analytica-EBC method 13.10 (Analytica-EBC, 2007). The members of the sensory panel were asked to describe seven flavour attributes for the aroma (fruity/estery, alcoholic/solvent, DMS (dimethyl-sulphide)/cooked vegetables, worty, malty, oxidized/aged and sweet) and twelve flavour attributes for the taste (fruity/estery, alcoholic/solvent, DMS/cooked vegetables, worty, malty, oxidized/aged, bitter, sweet, sour, astringent, body linger). A score was assigned ranging from 0 to 9, with 0 to 3 indicating none, 4 to 6 medium and 7 to 9 high-intensity flavour.
Statistical Model and Analyses
The statistical analyses were performed on the data collected (Statgraphics Centurion XVI version 16.1.11, StatPoint Technologies, Inc., Warrenton, VA, USA). The significant variations between the different independent samples obtained by the two prototypes were discriminated against using an unpaired t-test, while differences between samples along the storage time were evaluated by analysis of variance (ANOVA p < 0.05).
Results and Discussion
Quality Parameters of Wort
The quality parameters of the experimental wort were as follows: extract = 8.02 ± 0.04°P, pH = 5.54 ± 0.17, colour = 8.80 ± 0.28 EBC-U (European Brewery Convention Units), fermentability = 53.0 ± 1.4%.
The extract value was low, as expected for the production of LAB. The pH value was within the standard range. The colour was pale, reflecting the used raw material. The fermentability was extremely low, far from the optimal values for regular beer (> 80%), because of the specific mashing program used to obtain a LAB.
Sugar Profile of Wort and Beer
S. ludwigii is a selected yeast widely used to produce low-alcohol beer because it is not able to metabolize maltose. Table 1 shows the sugar profile of the wort and the beer analysed by HPLC. The obtained wort was specific for LAB production, with an extract of 8.02°P, similar to other work where non-Saccharomyces yeasts were studied (Bellut et al., 2018). S. ludwigii was able to use fructose, glucose and sucrose, while it did not metabolize maltose and maltotriose, according to previous works (Bellut et al., 2018; De Francesco et al., 2015a, b). The yeast consumed 9.9 g/l of sugar at the end of fermentation, leading to the formation of about 0.6% v/v of alcohol, as indicated in Table 2 (quality parameters of LAB), an alcohol level within the limit of a LAB. Looking at the dextrins, a slight increase in maltotetraose and dextrins > D4 at the end of fermentation occurred. This might be due to partial hydrolysis of the residual starch present. As mentioned above, a specific mashing program with an isothermal stage at 75 °C was used, which is optimal only for α-amylases enzymes, that break down starch into high molecular weight dextrins, leaving some residual starch in the mash. As expected, mashing at 75° C led to a lower presence of maltose than the work of Bellut et al. (2018). In that case, in which classic multi-step mashing was followed (40 min at 50 °C, 20 min at 62 °C, 20 min at 72 °C and 5 min at 78 °C), the maltose was 26.60 g/l in a wort of 6.63°P. In the present work, the maltose was 22.38 g/l in a wort of 8.02°P, in agreement with a recent study on low-alcohol beer production (Adamenko et al., 2020). This lower amount of maltose could give a less sweet taste and wort-like flavour to the final beer, one of the most important off-flavour of low-alcohol beer obtained by restricted fermentations or by using maltose negative yeast (Bellut & Arendt, 2019).
Quality Parameters of LAB
The quality parameters of LAB are shown in Table 2.
The variation of the original extract (OE), apparent extract (AE), apparent degree of fermentation (ADF) and the alcohol content can be used as markers of the stability of the unpasteurized beer, where the presence of residual yeast could cause the decrease in extract and so the increase in ethanol. The optimum fermentation temperature of the S. ludwigii yeast strain used is 23 °C, a value far from the beer storage temperature studied in the present work, which was 4 ± 1 °C. Ale strains usually grow and ferment poorly at temperatures below 12 °C (Vidgren, 2010). Further, the fermentation of S. ludwigii is naturally stopped by the depletion of the fermentable sugars (Bellut et al., 2018). As can be seen in Table 2, OE and AE showed a statistically significant decrease after 90 days of storage. Consequently, ADF increased after 90 days, going from 12.25 to 12.80% for sample A and from 12.45 to 12.70% for sample B. The alcohol content showed the same trend. The beer had an alcohol content of 0.6% v/v in the first 60 days, 0.7% v/v after 90 days and 0.8% v/v after 120 days. Samples A and B showed no statistical differences in alcohol content. The activity of S. ludwigii was confirmed by the level of carbon dioxide, a yeast fermentation by-product. Carbon dioxide rose from 4.50 g/l in the fresh beer to 4.65 and 4.75 g/l respectively for sample A and B, after 120 days (Table 2).
The pH of beer is a crucial quality parameter. The monitoring of this value allows the brewer to understand problems by a simple and fast measurement. A lowering of the pH could show the presence of lactic bacteria. On the contrary, an increase indicates the probable yeast autolysis (Kulka, 1953). A constant pH value during storage, therefore, shows a stable product. No beer showed statistically significant changes in pH (Table 2), showing an absence of bacterial development and autolysis of S. ludwigii.
The colour increase is an important beer ageing indicator, mainly due to oxidation and consequent degradation of polyphenols and the formation of Maillard compounds during storage (Vanderhaegen et al., 2003). The oxidation of beer polyphenols causes an increase in colour. To escape this, the brewing industries try to avoid the intake of oxygen as much as possible during all stages of production (Kirsop, 1974). In this case, surprisingly, the two samples assessed did not show statistically significant differences, despite the significant difference in TPO content into the two samples. The colour values increased from a first amount of 6.20 and 6.35 for sample A and B, respectively, to 6.60 EBC in both cases (Table 2). This may be due to the reduced amount of polyphenols given the low original extract of wort associated with the storage at low temperatures.
Haze in beer is one of the most complex beer aspects under study for many years. Haze can be due to several factors, especially during beer storage (Steiner et al., 2010). When low molecular weight polyphenols interact with proteins, a “chill haze” (visible when a beer is chilled to 0 °C but dissolved in solution when the beer is warmed at 20 °C) is formed. Particle sizes range from 0.1 to 1.0 µm. Polyphenols, however, can polymerize during beer storage generating a “permanent haze” (present in beer even at 20 °C), with particles between 1 and 10 µm (Bamforth, 1999). Polymerization of polyphenols is promoted by oxidation or even by the formation of aldehydes, including acetaldehyde, which can react with polyphenols to form species that cross-link with proteins. The haze in this work increased fourfold after 120 days. Once again, unexpectedly, the two beers showed the same behaviour, despite the oxygen content. The increase in haze occurred could be due to yeast suspended cells, in agreement with the slight yeast metabolism during storage. Secondly, because of the stress conditions of the yeast cells (cold temperature, high carbon dioxide), a release of storage polysaccharide glycogen, which is similar to starch (Steiner et al., 2010), but also of carbohydrates and proteins from slight yeast autolysis could be occurred. The value of haze is 0.50 EBC for 60 days, then it raised up to 1.90 EBC after 120 days for both samples (Table 2). Anyway, the trained panel judged all beers as brilliant. This discrepancy between instrumental and visual appearance might be due to the invisible haze formation (Rice et al., 2017).
Oxygen, as widely proved, has harmful effects on the stability of beer. A high presence of oxygen in the bottle causes an increase in colour and haze and loss of aroma and taste (Barnette & Shellhammer, 2019). In this work, the influence of oxygen was studied by comparing a beer filled without oxygen in the headspace (sample A) and one with oxygen in the headspace (sample B). Sample A had an optimal value of DO, ranging from 15 and 34 ppb during storage, lower than 50 ppb, the recommended value by the brewing industry (O’Rourke, 2002). DO values of sample B, on the contrary, ranged from 898 to 2940 ppb. DO values fluctuated, starting from 2163 ppb in fresh beer, then dropped to 898 after 30 days, climbed back to 2940 after 90 days and finally dropped again to 2300 ppb after 120 days (Table 2). Looking at the DO trend, it seems marginally influenced by the reducing power of the yeast. This result is in agreement with other authors who found that the presence of yeast can reduce oxygen only when the amount is low (Derdelinckx et al., 1992). In their study, the yeast was able to consume a significant amount of oxygen in the first few days. After 30 days, the DO remained over 2000 ppb. The oxygen migrated from the headspace remained into the solution, thus becoming an oxidizing agent boosting the ageing of the beer. TPO is the sum of the headspace oxygen and the DO. In this work, the bottles were stored statically, a condition that disadvantaged the solubilization of oxygen (Jaskula-Goiris et al., 2019). There were no statistically significant differences in TPO content between 30 and 120 days of storage for sample B and between 60 and 120 days of storage for sample A (Table 2).
Foam formation is eased in liquids of low surface tension, as less energy needs to be spent to produce the foam. LAB is an ambiguous substrate for foaming. The fewer dissolved substances (fewer proteins, low amount of hop) there are, the less likely bubbles are formed. On the other hand, lower alcohol content leads to more intense foam formation (Evans & Bamforth, 2009; Evans & Sheehan, 2002; Lewis & Lewis, 2003; Liguori et al., 2015, 2018). The stability of beer foam did not show statistically different values throughout the 120 days of storage. The foam had persistence of 180 s (Table 2), lower than a normal beer foam stability, which is considered best when greater than 230 s.
Volatile Compounds of LAB
Table 3 shows the values of higher alcohols and esters. Among the higher alcohols identified in beer, the most important in terms of flavour are n-propanol, isobutanol, 2-methyl-1-butanol and 3-methyl-1-butanol because they induce “alcoholic” flavour and aroma (De Francesco et al., 2015a, b). As expected, the reduced metabolic activity of the yeast led to the formation of a low level of higher alcohols, in agreement with the amount found in other published papers (Bellut et al., 2018; Narziss et al., 1992). Surprisingly, the strain used in this research produced fewer higher alcohols than the strains already studied by De Francesco et al., where the sum of higher alcohols was twofold with respect to the present work (De Francesco et al., 2015a, b). Higher alcohol level remained steady for 60 days. Then, an increase from 27.00 to 29.21 mg/l and from 26.52 to 28.93 mg/l, respectively for samples A and B, occurred after 90 days. This result is mainly due to the increase in 3-methyl-1-butanol which rose from 6.59 to 9.02 in sample A and from 6.58 to 9.03 in sample B (Table 3), according to the slow yeast activity that reduced 3-methylbutanal to 3-methylbutanol as the last step of the Ehrlich pathway. The level of higher alcohols is, as expected, lower than in a regular beer brewed with an ale (Saccharomyces cerevisiae) yeast (Pires et al., 2014).
Esters are the key aromatic compounds of fermentation. Ethyl acetate and isoamyl acetate, the two esters present in greater quantities, give fruity and complexity to the beer. As for the higher alcohols, yeast produced a low quantity of esters. The values found in Table 3 agree with other works about the LAB resulting far from a bottom fermented beer and even more from a top fermented beer, where the sum of the main esters can be up to 30 mg/l (Pires et al., 2014; Troilo et al., 2020). However, the ester content found in the present research was higher than the one found by Bellut et al. using the same strain (SL 17). This difference could be due to the various times chosen to stop the fermentation, namely, 48 h for Bellut et al. and over a week in the present work. A statistically significant decrease in ester content occurred during first 30 days of storage. The decrease in ester content is a signal of the ageing of beer, often due to the release of esterases from yeast autolysis. Such esterase activity is strain-dependent and top-fermenting yeasts are more active than bottom-fermenting yeasts. The optimal temperature of activity for these enzymes in beer is between 15 and 20 °C (Neven et al., 1997; Vanderhaegen et al., 2003). Thus, the cold storage could have slowed this reduction in esters (Table 3).
Table 4 shows the values of aldehydes and vicinal diketones (VDK).
Aldehydes are key compounds in beer ageing and are generally counted as off-flavour (Marconi et al., 2016; Rossi et al., 2014). Therefore, their monitoring allows to understand the stability of the beer. Oxidation of higher alcohols, Strecker degradation of amino acids, aldol condensation and oxidation of unsaturated fatty acids are the possible metabolic pathways that cause the formation of aldehydes during beer ageing (Vanderhaegen et al., 2004; Verstrepen et al., 2003). Because of the low content of melanoidins, demonstrated by the low values of beer colour, direct oxidation of alcohol by molecular oxygen should not be occurred in the present work, according to Vanderhaegen et al. (2006). For this reason, the increase in aldehydes should be due to other metabolic ways. Concerning acetaldehyde, an increase in this compound can be used as a marker for oxidation processes in bottled beer, especially in presence of air in the headspace (Vanderhaegen et al., 2003). In the present work, the acetaldehyde increased during the storage in sample B, with high oxygen content in the headspace (Table 4). 2-Methylbutanal and 3-methylbutanal are derived Strecker degradation aldehydes, which are formed from either valine or leucine in presence of oxygen (Wietstock et al., 2016). This pathway could lead to the constant increase in these two aldehydes. In sample B (with oxygen), the formation of these 2 aldehydes was much greater, confirming the catalysing action of oxygen (Table 4).
The hydroperoxy acid degradation or oxidation can lead to the formation of trans-2 nonenal and hexanal from lipid hydroperoxides in aqueous systems. Both aldehydes were checked in the present research. These aldehydes mainly originate during wort production. The used mashing-in temperature, above 70 °C, was much higher than the optimum for lipoxygenases. Moreover, the wort density was only 8.02°P. For these reasons, the level of trans-2-nonenal was expected to be low. Instead, a high trans-2-nonenal and hexanal content was found in the experimental beers, and then it decreased during cold storage (Table 4). Trans-2-nonenal content was much higher than what was found in a recent study where a beer obtained by S. ludwigii was analysed (Andrés-Iglesias et al., 2016). Hexanal was present in a low amount, according to other works where fresh beers obtained by S. ludwigii were analysed (De Francesco et al., 2015a, b; Narziss et al., 1992). The poor reduction of nonenal and hexanal found in fresh beer was likely due to the limited fermentation according to other works (Montanari et al., 2008).
Methional, a Strecker aldehyde, (worty off-flavour, cooked potato-like) also contributes to the ageing of beer. Methional is considered the key compound of wort sensation (Piornos et al., 2020), the most common LAB off-flavour. In regular beer, methional is present in a low amount, with an average of 3 µg/l (Guedes de Pinho & Silva Ferreira, 2006), differently from the results of the present work, where its concentration was 10.37 and 11.4 µg/l in fresh beer, for sample A and B, respectively. The reduced metabolism of the yeast generated a poor reducing activity and therefore the methional was not reduced to the corresponding alcohol. However, during storage a reduction in methional occurred, reaching 5.93 and 6.89 µg/l (Table 4). The presence of oxygen caused a minor reduction in methional.
As for methional, the Strecker aldehyde phenylacetaldehyde (honey-like) decreased during storage (Table 4). Furfural, a product of Maillard and caramelization reactions (heat indicator), is an aldehyde having a broad concentration range. A study by Brenner and Khan (1976) reports an average of 24.7 µg/l on 12 tested pasteurized beers, but it can also be over 500 µg/l in an aged beer (Saison et al., 2010). The furfural, although it poorly contributes to the ageing character of beer because of its high perception threshold, is monitored as a marker of ageing, pasteurization and warm storage. In the present work, its value slightly increased during cold storage, passing from 20 to 26 µg/l. The two samples did not show statistically significant differences, proving that furfural formation is independent to the presence of oxygen (Table 4).
Overall, except for acetaldehyde, 2-methylbutanal, 3-methylbutanal and furfural, the other aldehydes decreased during storage, most likely due to the presence of yeast which is considered the most powerful carbonyl-affecting agent involved in brewing (Saison et al., 2010). The results allow to conclude that during storage with a yeast activity, as in this case, not only a reduction activity takes place but several compounds, like acetaldehyde and Strecker aldehydes, could also be formed, according to other published works (Perpète & Collin, 2000).
The vicinal diketones diacetyl and 2,3-pentanedione clearly increased during beer ageing. The increase in vicinal diketones was the same in both samples, proving that the oxygen did not influence the diketone formation (Table 4). Diacetyl content was lower than the threshold. Anyway, in a LAB the threshold could be much lower than in a regular beer (Saison et al., 2009).
Sensory Evaluation of LAB
Figures 1 and 2 show the aroma and taste profile of the LAB. Fruity/estery, alcoholic/solvent, fruity/citrusy and hop descriptors were low for all beer throughout the storage, as expected by the low amount of added hop and by the concentration of esters and higher alcohols far below the perception threshold (Table 3).
The DMS/cooked vegetable and worty descriptors were high in all beers both for aroma and taste (Figs. 1 and 2). The judges explained these descriptors as a cooked potato and worty. This could derive from the methional content, which surpasses the threshold in all samples (Table 4), according to other papers (Alfeo et al., 2021).
The malt descriptor may come from 2-methylbutanal and 3-methylbutanal (Ferreira & Guido, 2018). The judges explained the malty character to resemble the aroma and taste of bread and almonds for all beers. This could be due to the above-cited aldehydes, despite their level below the threshold (Table 4). However, Perpète and Collin (2000) stated that the perception threshold of aromatic compounds in LAB is lower than in regular beer, because of the absence of masking compounds.
The presence of oxygen, as expected, caused a worsening of the aroma and taste in sample B. The oxidized/aged descriptor, in fact, was judged as high only for sample B. Even, the judges did not find this defect in sample A after 120 days of storage (Figs. 1 and 2). In sample B, the oxidation leads to the formation of off-flavours expressed by the judges as wet rag and stale bread. Further, cardboard off-flavour was stated in young beer and then disappeared after 90 days in sample A (figure B), in agreement with the volatile compound analyses, where trans-2-nonenal decreased during storage (Table 4).
Sweet is a common feature of LAB. The judges confirmed this aspect during the tasting sessions. The residual extract contributed to give to the beer also body, which was judged as well perceivable in both beers. In general, the judges considered sample A better than B. Cold storage preserved the taste, while sample B was found to have aged notes already after 90 days of storage at 4 °C.
Conclusions
The high residual extract and the low concentration of masking compounds make LAB susceptible to spoilage and flavour deterioration; therefore, they should be pasteurized before being placed on the market. Pasteurization also contributes to get the flavour less pleasant. In this work, the stability of an unpasteurized and unfiltered LAB was checked and the influence of oxygen on the volatile and organoleptic profile was studied. The cold chain has proved to be a practical option for a small brewery that has the possibility to locally sell the beer. LAB remained stable throughout the 120 days of monitoring, with a slight increase in ethanol (0.2% v/v). The presence of yeast protected the beer from oxidation. However, to limit the oxygen level is particularly important for stability. In fact, given the extremely high value of oxygen in the headspace, the tasting panel noticed an early ageing of sample B. Although keeping the cold chain is not possible for large industries, a small brewery with local sales can guarantee this and therefore it can offer to the consumer a stable product. However, it is still necessary to improve the organoleptic characteristics. The authors will continue the development of fruit beers and the addition of hops in dry-hopping to improve this LAB weakness.
The results confirmed the partial protective activity of yeast against oxidation and its ability to reduce some aldehydes. However, it has been noted that when the oxygen in the headspace is high, the yeast cannot absorb them all and therefore the beer is oxidized even at low storage temperatures. Low storage temperatures and reduced amount of oxygen seem to be optimal ways to extend the shelf life of LAB for small and local breweries. Obviously, pasteurization continues to be the best way to stabilize LAB beers. Small craft breweries could get help by cold chain and by shortening the best-before.
Data Availability
All data generated or analysed during this study are included in this published article.
References
Adamenko, K., Kawa-Rygielska, J., & Kucharska, A. Z. (2020). Characteristics of Cornelian cherry sour non-alcoholic beers brewed with the special yeast Saccharomycodes ludwigii. Food Chemistry, 312(November 2019), 125968. https://doi.org/10.1016/j.foodchem.2019.125968
Alfeo, V., De Francesco, G., Sileoni, V., Blangiforti, S., Palmeri, R., Aerts, G., et al. (2021). Physicochemical properties, sugar profile, and non-starch polysaccharides characterization of old wheat malt landraces. Journal of Food Composition and Analysis, 102(May), 103997. https://doi.org/10.1016/j.jfca.2021.103997
Analytica-EBC. (2007). European Brewery Convention. Analytica-EBC (5th ed.). Germany: Fachverlag Hans Carl.
Andrés-Iglesias, C., Nešpor, J., Karabín, M., Montero, O., Blanco, C. A., & Dostálek, P. (2016). Comparison of carbonyl profiles from Czech and Spanish lagers: traditional and modern technology. LWT - Food Science and Technology, 66, 390–397. https://doi.org/10.1016/j.lwt.2015.10.066
Bamforth, C. W. (1999). Beer haze. Journal of the American Society of Brewing Chemists, 57(3), 81–90. https://doi.org/10.1094/ASBCJ-57-0081
Barnette, B. M., & Shellhammer, T. H. (2019). Evaluating the impact of dissolved oxygen and aging on dry-hopped aroma stability in beer. Journal of the American Society of Brewing Chemists, 77(3), 179–187. https://doi.org/10.1080/03610470.2019.1603002
Bellut, K., & Arendt, E. K. (2019). Chance and challenge: Non-saccharomyces yeasts in nonalcoholic and low alcohol beer brewing–A review. Journal of the American Society of Brewing Chemists, 77(2), 77–91. https://doi.org/10.1080/03610470.2019.1569452
Bellut, K., Michel, M., Zarnkow, M., Hutzler, M., Jacob, F., De Schutter, D., et al. (2018). Application of non-Saccharomyces yeasts isolated from kombucha in the production of alcohol-free beer. Fermentation, 4(3), 66. https://doi.org/10.3390/fermentation4030066
Brendel, S., Hofmann, T., & Granvogl, M. (2020). Dry-hopping to modify the aroma of alcohol-free beer on a molecular level - Loss and transfer of odor-active compounds. Journal of Agricultural and Food Chemistry, 68(32), 8602–8612. https://doi.org/10.1021/acs.jafc.0c01907
Brenner, M. W., & Khan, A. A. (1976). Furfural and beer color as indices of beer flavor deterioration. Journal of the American Society of Brewing Chemists, 34(1), 14–19. https://doi.org/10.1080/03610470.1976.12006178
Callejo, M. J., García Navas, J. J., Alba, R., Escott, C., Loira, I., González, M. C., & Morata, A. (2019). Wort fermentation and beer conditioning with selected non-Saccharomyces yeasts in craft beers. European Food Research and Technology, 245(6), 1229–1238. https://doi.org/10.1007/s00217-019-03244-w
De Francesco, G., Freeman, G., Lee, E., Marconi, O., & Perretti, G. (2014). Effects of operating conditions during low-alcohol beer production by osmotic distillation. Journal of Agricultural and Food Chemistry, 62(14), 3279–3286. https://doi.org/10.1021/jf405490x
De Francesco, G., Sannino, C., Sileoni, V., Marconi, O., Filippucci, S., Tasselli, G., & Turchetti, B. (2018). Mrakia gelida in brewing process: An innovative production of low alcohol beer using a psychrophilic yeast strain. Food Microbiology, 76(June), 354–362. https://doi.org/10.1016/j.fm.2018.06.018
De Francesco, G., Sileoni, V., Marconi, O., & Perretti, G. (2015a). Pilot plant production of low-alcohol beer by osmotic distillation. Journal of the American Society of Brewing Chemists, 73(1), 41–48. https://doi.org/10.1094/ASBCJ-2015-0112-01
De Francesco, G., Turchetti, B., Sileoni, V., Marconi, O., & Perretti, G. (2015b). Screening of new strains of Saccharomycodes ludwigii and Zygosaccharomyces rouxii to produce low-alcohol beer. Journal of the Institute of Brewing, 121(1), 113–121. https://doi.org/10.1002/jib.185
Derdelinckx, G., Vanderhasselt, B., Maudoux, M., & Dufour, J. P. (1992). Refermentation in bottles and kegs: a rigorous approach. Brauwelt Int, 2(2), 156–164.
Evans, D. E., & Sheehan, M. C. (2002). Don’t be fobbed off: The substance of beer foam - A review. Journal of the American Society of Brewing Chemists, 60(2), 47–57. https://doi.org/10.1094/ASBCJ-60-0047
Evans, D. E., & Bamforth, C. W. (2009). Beer foam: achieving a suitable head. In and G. G. S. C. W. Bamforth, I. Russell (Ed.), Handbook of alcoholic beverages: Beer, a quality perspective (pp. 1–60). Burlington: Elsevier. https://doi.org/10.1016/B978-0-12-669201-3.00001-4
Feilner, R., & Oehmichen, T. (2016). Flash pasteurisation of filtrated-and wheat beer with one heat holding tube: a process comparison with shortened heat-holding. BrewingScience, 69(9–10), 64–72.
Ferreira, M., & Guido, F. (2018). Impact of wort amino acids on beer flavour: a review. Fermentation, 4(2), 23. https://doi.org/10.3390/fermentation4020023
Floridi, S., Miniati, E., Montanari, L., & Fantozzi, P. (2001). Carbohydrate determination in wort and beer by HPLC-ELSD. Monatsschrift Für Brauwissenshaft, 9(10), 209–215.
Gernat, D. C., Brouwer, E., & Ottens, M. (2020). Aldehydes as wort off-flavours in alcohol-free beers—Origin and control. Food and Bioprocess Technology, 13(2), 195–216. https://doi.org/10.1007/s11947-019-02374-z
Guedes de Pinho, P., & Silva Ferreira, A. C. (2006). Role of Strecker aldehydes on beer flavour stability. In Flavour science: Recent advances and trends (pp. 529–532). https://doi.org/10.1016/S0167-4501(06)80125-1
Halama, R., Brož, P., Izák, P., Kačírková, M., Dientsbier, M., & Olšovská, J. (2019). Beer dealcoholization using pervaporation. Kvasny Prumysl, 65(2), 65–71. https://doi.org/10.18832/kp2019.65.65
Jackowski, M., & Trusek, A. (2018). Non-alcoholic beer production-An overview. Polish Journal of Chemical Technology, 20(4), 32–38. https://doi.org/10.2478/pjct-2018-0051
Jaskula-Goiris, B., De Causmaecker, B., De Rouck, G., Aerts, G., Paternoster, A., Braet, J., & De Cooman, L. (2019). Influence of transport and storage conditions on beer quality and flavour stability. Journal of the Institute of Brewing, 125(1), 60–68. https://doi.org/10.1002/jib.535
Kirsop, B. H. (1974). Oxygen in brewery fermentation. Journal of the Institute of Brewing, 80(3), 252–259. https://doi.org/10.1002/j.2050-0416.1974.tb03614.x
Kulka, D. D. (1953). Yeast autolysis and the biological stability of beers. Journal of the Institute of Brewing. https://doi.org/10.1002/j.2050-0416.1953.tb02718.x
Lewis, M. J., & Lewis, A. S. (2003). Correlation of beer foam with other beer properties. MBAA Technical Quarterly, 40(2), 114–124.
Liguori, L., De Francesco, G., Albanese, D., Mincione, A., Perretti, G., Di Matteo, M., & Russo, P. (2018). Impact of osmotic distillation on the sensory properties and quality of low alcohol beer. Journal of Food Quality, 2018, 1–11. https://doi.org/10.1155/2018/8780725
Liguori, L., De Francesco, G., Russo, P., Albanese, D., Perretti, G., & Di Matteo, M. (2015). Quality improvement of low alcohol craft beer produced by evaporative pertraction. Chemical Engineering Transactions, 43, 13–18. https://doi.org/10.3303/CET1543003
Liguori, L., De Francesco, G., Russo, P., Perretti, G., Albanese, D., & Di Matteo, M. (2016). Quality attributes of low-alcohol top-fermented beers produced by membrane contactor. Food and Bioprocess Technology, 9(1), 191–200. https://doi.org/10.1007/s11947-015-1612-y
Lund, M. N., Hoff, S., Berner, T. S., Lametsch, R., & Andersen, M. L. (2012). Effect of pasteurization on the protein composition and oxidative stability of beer during storage. Journal of Agricultural and Food Chemistry. https://doi.org/10.1021/jf303044a
Marconi, O., Rossi, S., Galgano, F., Sileoni, V., & Perretti, G. (2016). Influence of yeast strain, priming solution and temperature on beer bottle conditioning. Journal of the Science of Food and Agriculture, 96(12), 4106–4115. https://doi.org/10.1002/jsfa.7611
Marques, C., Dinis, L., Barreiros Mota, I., Morais, J., Ismael, S., Pereira-Leal, J. B., et al. (2022). Impact of beer and nonalcoholic beer consumption on the gut microbiota: A randomized, double-blind, controlled trial. Journal of Agricultural and Food Chemistry. https://doi.org/10.1021/acs.jafc.2c00587
MEBAK. (2013). Wort, beer, beer-based beverages: collection of brewing analysis methods of the Mitteleuropäische Brautechnische Analysenkommission. Technische Universität München Forschungszentrum für Brau-und Lebensmittelqualität Alte Akademie, Freising, Munich.
Meilgaard, M. C., Elizondo, A., & Moya, E. (1970). A study of carbonyl compounds in beer, part II. Flavour and flavour thresholds of aldehydes and ketones added to beer. MBAA Technical Quarterly, 7(3), 143–149.
Milani, E. A., Ramsey, J. G., & Silva, F. V. M. (2016). High pressure processing and thermosonication of beer: Comparing the energy requirements and Saccharomyces cerevisiae ascospores inactivation with thermal processing and modeling. Journal of Food Engineering. https://doi.org/10.1016/j.jfoodeng.2016.02.023
Montanari, L., Marconi, O., Mayer, H., & Fantozzi, P. (2008). Production of alcohol-free beer. In V. R. Preedy (Ed.), Beer in Health and Disease Prevention (pp. 61–75). Elsevier Inc., Burlington, MA.
Narziss, L., Miedaner, H., Kern, E., & Leibhard, M. (1992). Technology and composition of non-alcoholic beers. Processes Using Arrested Fermentation. Brauwelt International, 4, 396–410.
Neven, H., Delvaux, F., & Derdelinckx, G. (1997). Flavor evolution of top fermented beers. Techical Quarterly, 34(2), 115–118. https://doi.org/10.1094/TQ-34
Nunes, B. V., da Silva, C. N., Bastos, S. C., & de Souza, V. R. (2022). Microbiological inactivation by ultrasound in liquid products. Food and Bioprocess Technology, 15(10), 2185–2209. https://doi.org/10.1007/s11947-022-02818-z
O’Rourke, T. (2002). The role of oxygen in brewing. Brewer International, (March), 45–47.
Peña-Gómez, N., Ruiz-Rico, M., Pérez-Esteve, É., Fernández-Segovia, I., & Barat, J. M. (2020). Microbial stabilization of craft beer by filtration through silica supports functionalized with essential oil components. Lwt, 117(September 2019), 108626. https://doi.org/10.1016/j.lwt.2019.108626
Perpète, P., & Collin, S. (2000). Influence of beer ethanol content on the wort flavour perception. Food Chemistry, 71(3), 379–385. https://doi.org/10.1016/S0308-8146(00)00179-5
Petrucci, R., Di Matteo, P., Sobolev, A. P. A. P., Liguori, L., Albanese, D., Proietti, N., et al. (2021). Impact of dealcoholization by osmotic distillation on metabolic profile, phenolic content, and antioxidant capacity of low alcoholic craft beers with different malt compositions. Journal of Agricultural and Food Chemistry, 69(16), acs.jafc.1c00679. https://doi.org/10.1021/acs.jafc.1c00679
Piornos, J. A., Balagiannis, D. P., Methven, L., Koussissi, E., Brouwer, E., & Parker, J. K. (2020). Elucidating the odor-active aroma compounds in alcohol-free beer and their contribution to the worty flavor. Journal of Agricultural and Food Chemistry, 68(37), 10088–10096. https://doi.org/10.1021/acs.jafc.0c03902
Pires, E. J., Teixeira, J. A., Brányik, T., & Vicente, A. A. (2014). Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Applied Microbiology and Biotechnology, 98, 1937–1949. https://doi.org/10.1007/s00253-013-5470
Rice, C. J., Pawlowsky, K., & Smart, C. (2017). Evaluating haze formation in flavoured lager beers using a range of forcing methods. Journal of the Institute of Brewing, 123(3), 388–395. https://doi.org/10.1002/jib.442
Rossi, S., Sileoni, V., Perretti, G., & Marconi, O. (2014). Characterization of the volatile profiles of beer using headspace solid-phase microextraction and gas chromatography-mass spectrometry. Journal of the Science of Food and Agriculture, 94(5), 919–928. https://doi.org/10.1002/jsfa.6336
Saison, D., De Schutter, D. P., Uyttenhove, B., Delvaux, F., & Delvaux, F. R. (2009). Contribution of staling compounds to the aged flavour of lager beer by studying their flavour thresholds. Food Chemistry, 114(4), 1206–1215. https://doi.org/10.1016/j.foodchem.2008.10.078
Saison, D., De Schutter, D. P., Vanbeneden, N., Daenen, L., Delvaux, F., & Delvaux, F. R. (2010). Decrease of aged beer aroma by the reducing activity of brewing yeast. Journal of Agricultural and Food Chemistry, 58(5), 3107–3115. https://doi.org/10.1021/jf9037387
Shen, Y., Liu, C., Yin, X., Peng, L., & Li, Q. (2014). Influence of pasteurization and microfiltration on beer aging and anti-aging levels. Journal of the American Society of Brewing Chemists, 72(4), 285–295. https://doi.org/10.1094/ASBCJ-2014-0925-01
Steiner, E., Becker, T., & Gastl, M. (2010). Turbidity and haze formation in beer—Insights and overview. Journal of the Institute of Brewing, 116(4), 360–368. https://doi.org/10.1002/j.2050-0416.2010.tb00787.x
Tomasi, I., Marconi, O., Sileoni, V., & Perretti, G. (2017). Validation of a high-performance size-exclusion chromatography method to determine and characterize β-glucans in beer wort using a triple-detector array. Food Chemistry. https://doi.org/10.1016/j.foodchem.2016.06.092
Troilo, A., De Francesco, G., Marconi, O., Sileoni, V., Turchetti, B., & Perretti, G. (2020). Low carbohydrate beers produced by a selected yeast strain from an alternative source. Journal of the American Society of Brewing Chemists, 78(1), 80–88. https://doi.org/10.1080/03610470.2019.1682887
Vanderhaegen, B., Neven, H., Coghe, S., Verstrepen, K. J., Verachtert, H., & Derdelinckx, G. (2003). Evolution of chemical and sensory properties during aging of top-fermented beer. Journal of Agricultural and Food Chemistry, 51(23), 6782–6790. https://doi.org/10.1021/jf034631z
Vanderhaegen, B., Neven, H., Verachtert, H., & Derdelinckx, G. (2006). The chemistry of beer aging – A critical review. Food Chemistry, 95(3), 357–381. https://doi.org/10.1016/j.foodchem.2005.01.006
Vanderhaegen, B., Neven, H., Verstrepen, K. J., Delvaux, F. R., Verachtert, H., & Derdelinckx, G. (2004). Influence of the brewing process on furfuryl ethyl ether formation during beer aging. Journal of Agricultural and Food Chemistry, 52(22), 6755–6764. https://doi.org/10.1021/jf0490854
Varela, J., & Varela, C. (2019). Microbiological strategies to produce beer and wine with reduced ethanol concentration. Current Opinion in Biotechnology, 56, 88–96. https://doi.org/10.1016/j.copbio.2018.10.003
Verstrepen, K. J., Van Laere, S. D. M., Vanderhaegen, B. M. P., Derdelinckx, G., Dufour, J. P., Pretorius, I. S., et al. (2003). Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of volatile esters. Applied and Environmental Microbiology, 69(9), 5228–5237. https://doi.org/10.1128/AEM.69.9.5228-5237.2003
Vidgren, V. (2010). Maltose and maltotriose transport into ale and lager brewer’s yeast strains. Espoo 2010. VTT Publications 748. 93 p. A dissertation for the degree of Doctor of Philosophy, University of Helsinki, Main Building, Unioninkatu 34, 10th of December.
Wietstock, P. C., Kunz, T., & Methner, F. J. (2016). Relevance of oxygen for the formation of Strecker aldehydes during beer production and storage. Journal of Agricultural and Food Chemistry, 64(42), 8035–8044. https://doi.org/10.1021/acs.jafc.6b03502
Acknowledgements
Thanks are due to Dr Elio Moretti to produce wort and beers and to Dr Simona Floridi for the coordination of the analyses.
Funding
Open access funding provided by Università degli Studi di Perugia within the CRUI-CARE Agreement. This work was supported by POR FSE Umbria 2014–2020—Asse 3, Perugia, Italy.
Author information
Authors and Affiliations
Contributions
Conceptualization: Ombretta Marconi and Giovanni De Francesco; data curation: Stefano Maranghi and Valeria Sileoni; funding acquisition: Ombretta Marconi and Giuseppe Perretti; investigation: Giovanni De Francesco, Valeria Sileoni and Stefano Maranghi; methodology: Giovanni De Francesco and Ombretta Marconi; supervision: Ombretta Marconi and Giuseppe Perretti; validation: Ombretta Marconi and Valeria Sileoni; roles/writing—original draft: Giovanni De Francesco and Stefano Maranghi; writing—review and editing: Giovanni De Francesco, Valeria Sileoni, Ombretta Marconi.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sileoni, V., Maranghi, S., De Francesco, G. et al. Flavour Stability of a Cold-Stored Unpasteurized Low-Alcohol Beer Produced by Saccharomycodes ludwigii. Food Bioprocess Technol 16, 2471–2482 (2023). https://doi.org/10.1007/s11947-023-03061-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03061-w