Fractional distillation of crude oil (GCSE)
Industrial Fractional Distillation of Crude Oil
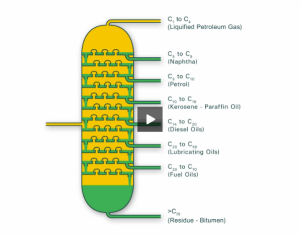
This is carried out in oil refineries using giant fractionating columns (also known as fractionating towers). These are often located in close proximity to the crude oil source.
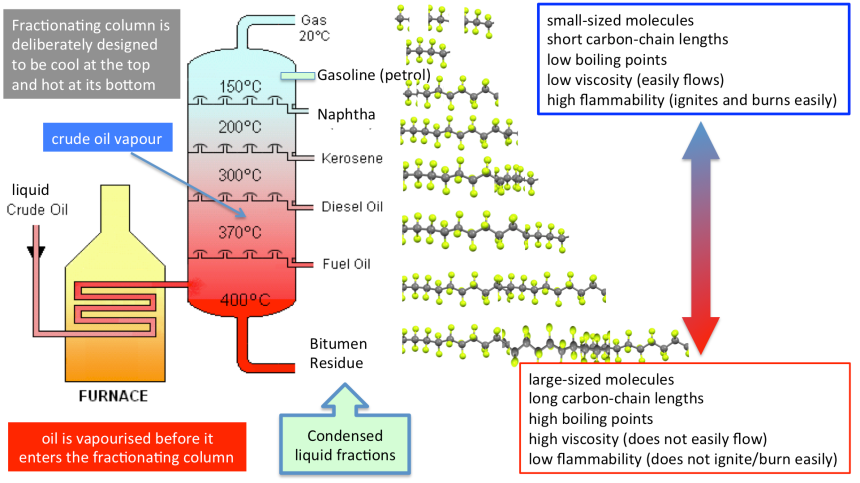
The industrial fractionating column is designed to be deliberately cool at the top and hot at its base– this means that the industrial fractionating column is able to cool and condense the crude oil vapour at distinctly different temperature ranges that have been established by the column’s temperature gradient.
Hence, the industrial fractionating column is acting as a highly specialised condenser of the crude oil vapour.
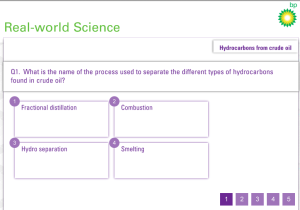
Industrial fractional distillation of crude oil generates fractions that have distinctly different boiling points, properties and uses. How is this process achieved?
This is a classic 6 mark QWC (Quality of written communication) exam-style question!!! I will answer this showing you where I believe the 6 marks exist.
- The industrial fractionating column is designed to be deliberately cool at the top and hot at its base.
- Crude oil is heated to 400C to ensure complete vapourisation of all hydrocarbons present with the crude oil mixture.
- Crude oil vapour is pumped into the base of the fractionating column, the vapur then begins to rise up the column and cool. At different heights within the column, hydrocarbons will condense to liquid if they reach a temperature that is at, or just below, their boiling point.
4. Large-sized hydrocarbons, with long carbon-chain lengths, will cool and condense to form liquid fractions at the base of the column where the temperature is hot and fixed to be at, or just below, their boiling point.
5. The crude oil vapour will no longer contain larger-sized hydrocarbons and only contain the remaining smaller-sized hydrocarbon molecules. This partially distilled vapour will continue to rise, cool and condense within the fractionating column.
6. Small-sized hydrocarbons, with short carbon-chain lengths, will cool and condense to form liquid fractions towards the top of the column where the temperature is cool and fixed to be at, or just below, their boiling point.
(There may be even better explanations out there, but the above is my attempt!)
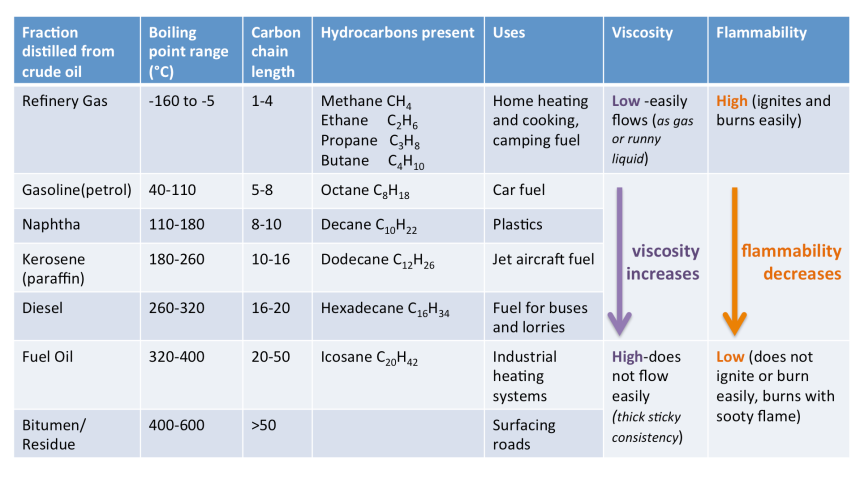
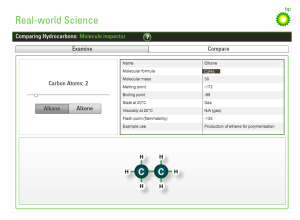
The two diagrams below illustrate the discussed mechanism. The latter diagram depicts a table that highlights the differences in the boiling point range, carbon-chain length, viscosity, flammability and use for each crude oil fraction.
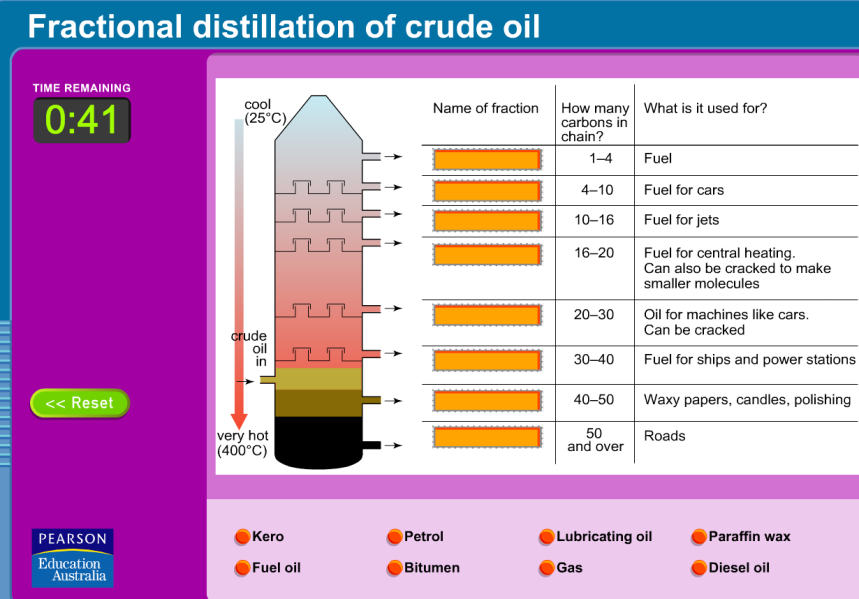
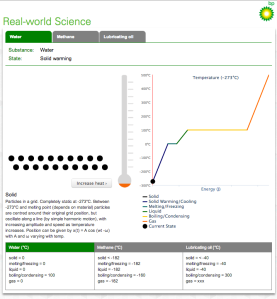
There are four excellent interactive simulations that illustrate the theory and test your understanding.
For more advanced information, the following resources by BP education are particularly informative.