The non-invasive prenatal test is a diagnostic test that analyzes the DNA of the fetus found in a maternal blood sample.
Its purpose is to predict the baby's risk of having certain chromosomal abnormalities, such as Down syndrome or Edwards syndrome, but with the advantage of not being an invasive test that endangers pregnancy.
Therefore, the maternal blood fetal DNA test is an increasingly used alternative to the classical prenatal studies, i.e. amniocentesis and chorionic villus sampling, both associated with a high risk of gestational loss.
Provided below is an index with the 7 points we are going to expand on in this article.
- 1.
- 1.1.
- 1.2.
- 2.
- 3.
- 4.
- 4.1.
- 4.2.
- 4.3.
- 4.4.
- 4.5.
- 5.
- 6.
- 7.
What is non-invasive prenatal testing?
The maternal blood fetal DNA test is a non-invasive diagnostic test that allows the genetic material of the fetus to be studied by obtaining fetal DNA from the mother's blood.
First, the DNA of the fetus must be separated from that of the mother and then sequenced in the laboratory to find out if there is any genetic alteration.
Between 4 and 10% of the free DNA found in a pregnant woman's blood plasma is from the fetus, probably of placental origin.
The presence of fetal DNA in the mother's bloodstream can be detected as early as the 9th week of pregnancy. It comes from the apoptosis (programmed cell death) of the fetal cells and will disappear a few hours after delivery.
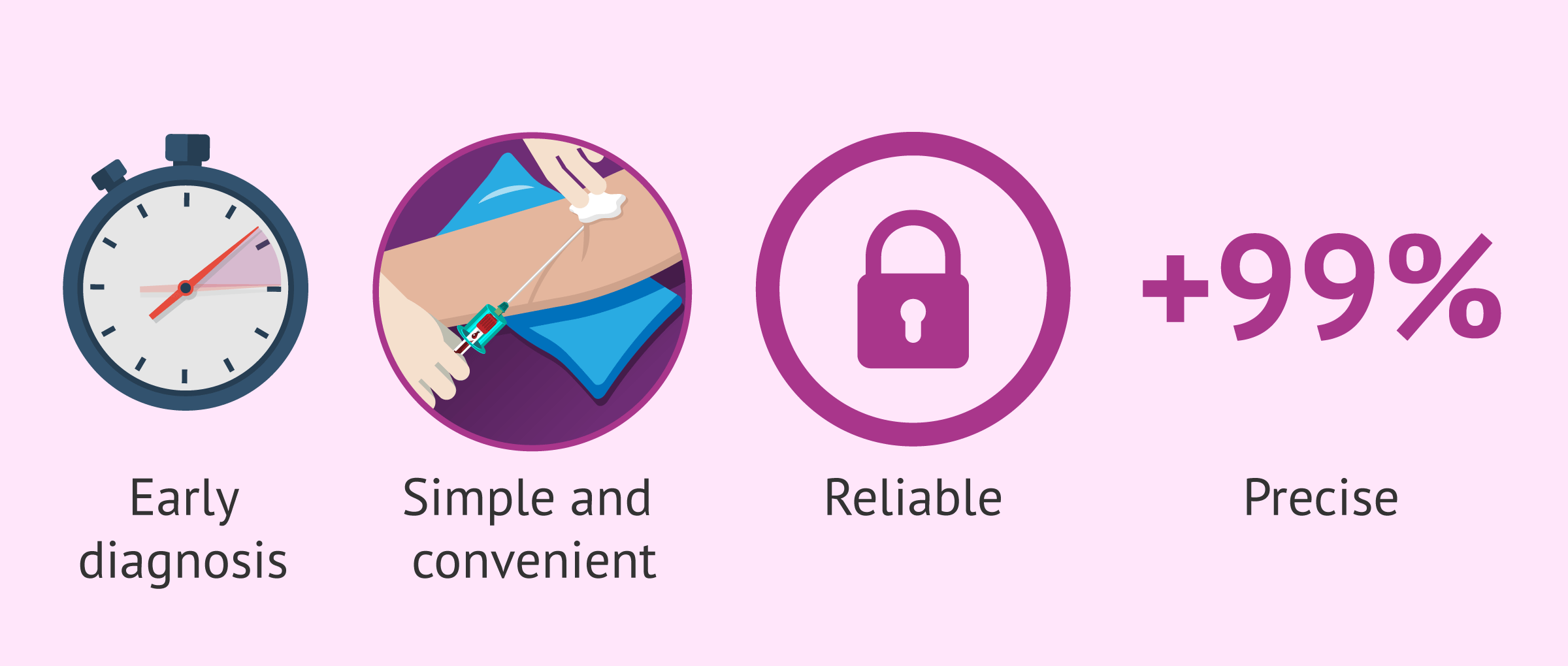
Advantages
The advantages of non-invasive antenatal testing are multiple compared to other antenatal tests such as combined first trimester screening or amniocentesis. We will discuss them below:
- Early diagnosis
- from the 10th week of pregnancy the test can be performed reliably.
- Simplicity and comfort
- only a blood sample from the mother obtained as in a routine analysis is necessary.
- Rapidity
- the results can be obtained between 3 and 15 days depending on the test and the laboratory where it is performed.
- Security
- there is no risk to the mother or the fetus.
- Accuracy
- its detection rate is above 99%, compared to 90% for triple screening. In addition, its false-positive rate is less than 0.1%, while that of triple screening is 5%.
The latter means that the maternal blood fetal DNA test is much more reliable than the combined first trimester screening, which reduces the number of unnecessary amniocentesis.
Limitations
Since this is a fairly recent diagnostic test, the non-invasive prenatal test still has some limitations that make it impossible to replace invasive tests definitively. In the following section we are going to to comment on these limitations:
- Although it detects the most frequent chromosomal alterations, the maternal blood fetal DNA test is not useful for detecting any genetic anomaly. It still has limited diagnostic ability.
- It is also unable to detect fetal malformations and is therefore no substitute for morphological ultrasound.
- Its accuracy is very high, but it's still a screening test. If a positive result is obtained, it will always be confirmed with an amniocentesis or chorionic biopsy.
- Costs Non-invasive prenatal testing is expensive. Please make sure to contact your health insurance if the treatment is covered.
- There is a risk greater than 5% of not obtaining results in obese patients.
- In case of twin pregnancy, it is not valid for analyzing the sex chromosomes.
Indications of fetal DNA testing
Any pregnant woman can undergo this non-invasive test to know the status of her baby and follow pregnancy more relaxed.
However, the maternal blood fetal DNA test is particularly indicated in the following cases:
- Combined first-trimester screening results in a high risk of chromosomal alterations.
- When there is a history of previous pregnancy with abnormalities.
- The karyotype of either parent is altered.
- Suspicious results on the 12-week ultrasound.
Despite its high cost, the non-invasive prenatal test has managed to reduce unnecessary amniocentesis by approximately 90%, confirming the non-existence of the chromosomal alterations initially indicated by triple screening.
What genetic alterations can be detected?
Currently, there are two types of non-invasive prenatal testing on the market: basic and advanced or extended. The latter makes it possible to analyse a larger number of chromosomes.
The basic fetal DNA test analyses the most frequent chromosomal alterations in the population, which are the following:
- Down syndrome (trisomy 21)
- Edwards syndrome (trisomy 18)
- Patau syndrome (trisomy 13)
- Aneuploidy on sex chromosomes: Turner syndrome (monosomies X0), Klinefelter syndrome (trisomy XXY), etc.
In addition to these chromosomal abnormalities, it is also possible to detect the sex of the future baby.
As for the advanced or extended non-invasive prenatal test, in addition to the above-mentioned alterations, it also identifies mutations related to the following diseases:
- DiGeorge Syndrome
- Deletion Syndrome 1p36
- Angelman syndrome
- Prader-Willi syndrome
- Cri du chat snydrome
- Wolf-Hirschhorn syndrome
Finally, it is important to point out that, although the non-invasive prenatal study has been a great advance, there are pathologies that can only be detected when invasive tests such as amniocentesis or chorionic villus biopsy are carried out, so these are not completely out of use.
FAQs from users
Is it advisable to test maternal blood if I have already done PGD?
The non-invasive prenatal test is a prenatal test that is performed on maternal blood and allows the detection of the most frequent chromosomal abnormalities (Down syndrome and other trisomies). This genetic analysis has a sensitivity close to 95% from 10 weeks of gestation.
If the patient has undergone assisted reproduction treatment in which PGD has been performed, the fetal chromosomes have been studied directly. For this reason, the study for the diagnosis of aneuploidy with another method would not be necessary
Which women should have a cell-free fetal dna testing performed?
Ideally, fetal DNA testing should be the method used in all pregnant women. However, most health care providers only cover the costs for high-risk patients.
In general, we could say that this test would be especially recommended in the following groups of women:
- Women who underwent first trimester screening and are considered high-risk patients(≥1/270)
- Women who presented aneuploidies in chromosomes 21, 18 or 13 in their previous pregnancy.
- Women who are pregnant at ≥ 38 years.
How is it possible to separate fetal DNA from maternal DNA?
Separating the DNA of the fetus from the DNA of the mother is one of the main limitations of this test, as it is not entirely uncomplicated.
If the fetus is male, it is possible to identify the Y chromosome sequences unique to the male sex and therefore it is easier to distinguish fetal DNA from maternal DNA.
However, when the fetus is a girl, the diagnosis is more complicated. In this case, the methods used to separate the fetal DNA from that of the mother are based on looking for differences in the methylation of the genes or in the length of the DNA fragments, as those from the fetus are shorter.
Finally, it should be noted that the most common way to distinguish the fetal from the maternal genetic endowment is to look for genetic characteristics of the father inherited by the fetus and which are therefore not present in the mother's DNA.
When to do a non-invasive prenatal test?
Today, this non-invasive test is generally performed on pregnant women who obtain risk results from the first trimester triple screening study. The sensitivity obtained for the analysis of trisomies 21, 18 and 13 is greater than 99%.
However, any woman who wishes to do so can do so for peace of mind, even if it is not a risky pregnancy.
If the test were positive, it would be necessary to confirm it with invasive prenatal diagnostic techniques (amniocentesis and chorionic biopsy).
What is the price of non-invasive prenatal testing?
Depending on the laboratory where it is performed, the price of the fetal DNA test can vary between 500-600€ if it is the most basic test (trisomies 21, 18 and 13) and between 700-800€ if it is the extended test.
Suggested for you
As we have commented throughout this post, in the event of a positive result in the fetal DNA test in maternal blood, it will be necessary to confirm it through an invasive prenatal test such as amniocentesis. You can read more about this here: Indications and risks of amniocentesis.
As for the other invasive prenatal test used, chorion biopsy, you can learn more about it in the following article: What is chorionic villus sampling? - Indications and risks
We make a great effort to provide you with the highest quality information.
🙏 Please share this article if you liked it. 💜💜 You help us continue!
References
Ioan Dumitru Suciu, Oana Daniela Toader, Slavyana Galeva, Lucian Pop. Non-Invasive Prenatal Testing beyond Trisomies. J Med Life. 2019 Jul-Sep;12(3):221-224. doi: 10.25122/jml-2019-0053 (View)
Lene K. Juvet, Sari S. Ormstad, Anna Stoinska‐Schneider, Berge Solberg, Helene Arentz‐Hansen, Maria Knoph Kvamme, Brynjar Fure. Non-Invasive Prenatal Test (NIPT) for Identification of Trisomy 21, 18 and 13. Oslo, Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2016 Dec 16. Report from the Norwegian Institute of Public Health No. 2016-18 (View)
Luigi Carbone, Federica Cariati, Laura Sarno, Alessandro Conforti, Francesca Bagnulo, Ida Strina, Lucio Pastore, Giuseppe Maria Maruotti, Carlo Alviggi. Non-Invasive Prenatal Testing: Current Perspectives and Future Challenges. Genes (Basel). 2020 Dec 24;12(1):15. doi: 10.3390/genes12010015 (View)
Mylène Badeau, Carmen Lindsay, Jonatan Blais, Leon Nshimyumukiza, Yemisi Takwoingi, Sylvie Langlois, France Légaré, Yves Giguère, Alexis F Turgeon, William Witteman, François Rousseau. Genomics-based non-invasive prenatal testing for detection of fetal chromosomal aneuploidy in pregnant women. Cochrane Database Syst Rev. 2017 Nov 10;11(11):CD011767 (View)
P Mohan, J Lemoine, C Trotter, I Rakova, P Billings, S Peacoc, C-Y Kao, Y Wang, F Xia, C M Eng, P Benn. Clinical experience with non-invasive prenatal screening for single-gene disorders. Ultrasound Obstet Gynecol. 2022 Jan;59(1):33-39. doi: 10.1002/uog.23756 (View)
Siva Shantini Jayashankar, Muhammad Luqman Nasaruddin, Muhammad Faiz Hassan, Rima Anggrena Dasrilsyah, Mohamad Nasir Shafiee, Noor Akmal Shareela Ismail, Ekram Alias. Non-Invasive Prenatal Testing (NIPT): Reliability, Challenges, and Future Directions. Diagnostics (Basel). 2023 Aug 2;13(15):2570. doi: 10.3390/diagnostics13152570 (View)
FAQs from users: 'Is it advisable to test maternal blood if I have already done PGD?', 'Which women should have a cell-free fetal dna testing performed?', 'How is it possible to separate fetal DNA from maternal DNA?', 'When to do a non-invasive prenatal test?' and 'What is the price of non-invasive prenatal testing?'.