Development and Characterization of Probiotic Beers with Saccharomyces boulardii as an Alternative to Conventional Brewer’s Yeast
Abstract
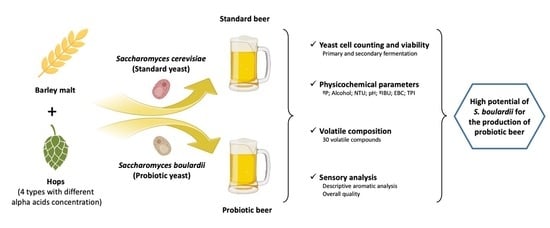
:1. Introduction
2. Materials and Methods
2.1. Inoculum Preparation
2.2. Beer-Making Procedure
2.2.1. Primary Fermentation
2.2.2. Secondary Fermentation and Maturation
2.3. Yeast Cell Counting and Viability
2.4. Physical-Chemical Analysis
2.5. Analysis of Volatile Compounds
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Yeast Cell Counting and Viability
3.2. Beer Characterization after Secondary Fermentation and Maturation
3.2.1. Yeast Cell Counting and Viability
3.2.2. Physicochemical Parameters
3.2.3. Volatile Profile
3.2.4. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sadler, J. Innovation in Functional Food and Drinks; Bus. Insights Ltd.: Warwick, UK, 2005. [Google Scholar]
- Fernandes, S.S.; Coelho, M.S.; de las Mercedes Salas-Mellado, M. Bioactive Compounds as Ingredients of Functional Foods. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 129–142. [Google Scholar]
- Khan, R.S.; Grigor, J.; Winger, R.; Win, A. Functional food product development—Opportunities and challenges for food manufacturers. Trends Food Sci. Technol. 2013, 30, 27–37. [Google Scholar] [CrossRef]
- Markets and Markets Dairy Alternatives Market by Source (Soy, Almond, Coconut, Rice, Oats, Hemp), Application (Milk, Cheese, Yogurt, Ice Creams, Creamers), Distribution Channel (Supermarkets, Health Stores, Pharmacies), Formulation and Region—Forecast to 2027. 2022. Available online: https://www.researchandmarkets.com/reports/5239110/dairy-alternatives-market-by-source-soy (accessed on 10 July 2023).
- FAO/WHO. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Lorenzo Morelli, R.B.C.; Flint, H.J.; Salminen, S.; Calder, P.C.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Lokhande, S.; More, S.; Raje, V. A Systematic Study of Probiotics—An Update Review. Asian J. Pharm. Technol. 2018, 8, 149–157. [Google Scholar] [CrossRef]
- McFarland, L. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J. Gastroenterol. 2010, 16, 2202–2222. [Google Scholar] [CrossRef]
- Ohland, C.; Macnaughton, W. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef] [Green Version]
- Czerucka, D.; Piche, T.; Rampal, P. Review article: Yeast as probiotics—Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26, 767–778. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Abid, R.; Waseem, H.; Ali, J.; Ghazanfar, S.; Ali, G.M.; Elasbali, A.M.; Alharethi, S.H. Probiotic Yeast Saccharomyces: Back to Nature to Improve Human Health. J. Fungi 2022, 8, 444. [Google Scholar] [CrossRef]
- Lillo-Pérez, S.; Guerra-Valle, M.; Orellana-Palma, P.; Petzold, G. Probiotics in fruit and vegetable matrices: Opportunities for nondairy consumers. LWT 2021, 151, 112106. [Google Scholar] [CrossRef]
- Mantzourani, I.; Kazakos, S.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Bekatorou, A.; Plessas, S. Potential of the probiotic Lactobacillus plantarum ATCC 14917 strain to produce functional fermented pomegranate juice. Foods 2019, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Rodhouse, L.; Carbonero, F. Overview of craft brewing specificities and potentially associated microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 462–473. [Google Scholar] [CrossRef]
- Euromonitor International. What’s Brewing in Craft Beer? Euromonitor International: Hong Kong, China, 2019. [Google Scholar]
- Senkarcinova, B.; Graça Dias, I.A.; Nespor, J.; Branyik, T. Probiotic alcohol-free beer made with Saccharomyces cerevisiae var. boulardii. LWT 2019, 100, 362–367. [Google Scholar] [CrossRef]
- Yeo, H.Q.; Liu, S.-Q. An overview of selected specialty beers: Developments, challenges and prospects. Int. J. Food Sci. Technol. 2014, 49, 1607–1618. [Google Scholar] [CrossRef]
- Rošul, M.; Mandić, A.; Mišan, A.; Đerić, N.; Pejin, J. Review of trends in formulation of functional beer. Food Feed Res. 2019, 46, 23–35. [Google Scholar] [CrossRef]
- Ogueke, C.C.; Owuamanam, C.I.; Ihediohanma, N.C.; Iwouno, J.O. Probiotics and Prebiotics: Unfolding Prospects for Better Human Health. Pak. J. Nutr. 2010, 9, 833–843. [Google Scholar] [CrossRef] [Green Version]
- European Brewery Convention Method. 9.2.1—Alcohol in Beer by Distillation. In Analytica-EBC; Schweizer Brauerei-Rundschau: Celerina, Switzerland, 1987. [Google Scholar]
- European Brewery Convention Method. 9.6—Colour of beer: Spectrophotometric method. In Analytica-EBC; Schweizer Brauerei-Rundschau: Celerina, Switzerland, 1987. [Google Scholar]
- Ruvalcaba, J.E.; Durán-Guerrero, E.; Barroso, C.G.; Castro, R. Development of a stir bar sorptive extraction method to study different beer styles volatile profiles. Food Res. Int. 2019, 126, 108680. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae var. boulardii in co-fermentations with S. cerevisiae for the production of craft beers with potential healthy value-added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef]
- Vriesekoop, F.; Krahl, M.; Hucker, B.; Menz, G. 125th Anniversary review: Bacteria in brewing: The good, the bad and the ugly. J. Inst. Brew. 2012, 118, 335–345. [Google Scholar] [CrossRef]
- Alcine Chan, M.Z.; Chua, J.Y.; Toh, M.; Liu, S.Q. Survival of probiotic strain Lactobacillus paracasei L26 during co-fermentation with S. cerevisiae for the development of a novel beer beverage. Food Microbiol. 2019, 82, 541–550. [Google Scholar] [CrossRef]
- Beer Judge Certification Program. 2015. Available online: https://www.bjcp.org/style/2015/ (accessed on 10 July 2023).
- Bokulich, N.A.; Bamforth, C.W. The Microbiology of Malting and Brewing. Microbiol. Mol. Biol. Rev. 2013, 77, 157–172. [Google Scholar] [CrossRef] [Green Version]
- Pereira, B.; Paula, D.; de Souza, H.; Firmino, L.; Jos, W.; Signori, K.; Alice, M.; Coelho, Z. Technological features of Saccharomyces cerevisiae var. boulardii for potential probiotic wheat beer development. LWT 2021, 135, 110233. [Google Scholar] [CrossRef]
- Mulero-Cerezo, J.; Briz-Redón, Á.; Serrano-Aroca, Á. Saccharomyces cerevisiae Var. Boulardii: Valuable Probiotic Starter for Craft Beer Production. Appl. Sci. 2019, 9, 3250. [Google Scholar] [CrossRef] [Green Version]
- Sohrabvandi, S.; Razavi, S.H.; Mousavi, S.M.; Amir, M. Viability of probiotic bacteria in low alcohol- and non- alcoholic beer during refrigerated storage. Philipp. Agric. Sci. 2010, 93, 24–28. [Google Scholar]
- Mortazavian, A.; Sohrabvandi, S. Probiotic products. In Probiotics and Food Probiotic Products Based on Dairy Probiotic Products; Eta Publication: Tehran, Iran, 2006; pp. 330–372. [Google Scholar]
- Liu, S.-Q. Impact of yeast and bacteria on beer appearance and flavour. In Brewing Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 357–374. [Google Scholar]
- Collins, M.A.; Neafsey, E.J.; Mukamal, K.J.; Gray, M.O.; Parks, D.A.; Das, D.K.; Korthuis, R.J. Alcohol in Moderation, Cardioprotection, and Neuroprotection: Epidemiological Considerations and Mechanistic Studies. Alcohol. Clin. Exp. Res. 2009, 33, 206–219. [Google Scholar] [CrossRef] [Green Version]
- Bortoleto, G.G.; Gomes, W.P.C.; Ushimura, L.C.; Bonanca, R.A.; Novello, E.H. Evaluation of the profile of volatile organic compounds in industrial and craft beers. J. Microbiol. Biotechnol. Food Sci. 2022, 12, e5532. [Google Scholar] [CrossRef]
- Salanță, L.C.; Coldea, T.E.; Ignat, M.V.; Pop, C.R.; Tofană, M.; Mudura, E.; Borșa, A.; Pasqualone, A.; Zhao, H. Non-alcoholic and craft beer production and challenges. Processes 2020, 8, 1382. [Google Scholar] [CrossRef]
- Reitenbach, A.F.; Iwassa, I.J.; Barros, B. Production of functional beer with the addition of probiotic: Saccharomyces boulardii. Res. Soc. Dev. 2021, 10, e5010212211. [Google Scholar] [CrossRef]
- Bamforth, C. pH in brewing: An overview. Tech. Q. Master Brew. Assoc. Am. 2001, 38, 1–9. [Google Scholar]
- Silva, S.; Oliveira, A.I.; Cruz, A.; Oliveira, R.F.; Almeida, R.; Pinho, C. Physicochemical Properties and Antioxidant Activity of Portuguese Craft Beers and Raw Materials. Molecules 2022, 27, 8007. [Google Scholar] [CrossRef]
- Reinoso Carvalho, F.; Wang, Q.; de Causmaecker, B.; Steenhaut, K.; van Ee, R.; Spence, C. Tune That Beer! Listening for the Pitch of Beer. Beverages 2016, 2, 31. [Google Scholar] [CrossRef] [Green Version]
- Baiano, A.; Fiore, A.; la Gatta, B.; Tufariello, M.; Gerardi, C.; Savino, M.; Grieco, F. Single and Interactive Effects of Unmalted Cereals, Hops, and Yeasts on Quality of White-Inspired Craft Beers. Beverages 2023, 9, 9. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Pérez-Jiménez, J. What Contribution Is Beer to the Intake of Antioxidants in the Diet? In Beer in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2009; pp. 441–448. [Google Scholar]
- Oladokun, O.; Tarrega, A.; James, S.; Smart, K.; Hort, J.; Cook, D. The impact of hop bitter acid and polyphenol profiles on the perceived bitterness of beer. Food Chem. 2016, 205, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef]
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shimizu, H.; Shioya, S. Beer Volatile Compounds and Their Application to Low-Malt Beer Fermentation. J. Biosci. Bioeng. 2008, 106, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Olaniran, A.O.; Hiralal, L.; Mokoena, M.P.; Pillay, B. Flavour-active volatile compounds in beer: Production, regulation and control. J. Inst. Brew. 2017, 123, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [Green Version]
- Fracassetti, D.; Bottelli, P.; Corona, O.; Foschino, R.; Vigentini, I. Innovative Alcoholic Drinks Obtained by Co-Fermenting Grape Must and Fruit Juice. Metabolites 2019, 9, 86. [Google Scholar] [CrossRef] [Green Version]
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.-P.; Winderickx, J.; Thevelein, J.M.; Pretorius, I.S.; Delvaux, F.R. Flavor-active esters: Adding fruitiness to beer. J. Biosci. Bioeng. 2003, 96, 110–118. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Zannini, E.; Ciani, M.; Comitini, F. Lentil Fortification and Non-Conventional Yeasts as Strategy to Enhance Functionality and Aroma Profile of Craft Beer. Foods 2022, 11, 2787. [Google Scholar] [CrossRef]
- Villamor, R.R.; Ross, C.F. Wine Matrix Compounds Affect Perception of Wine Aromas. Annu. Rev. Food Sci. Technol. 2013, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.C.; Faria, M.A.; Ferreira, I.M.P.L.V.O. Hops: New Perspectives for an Old Beer Ingredient. In Natural Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 267–301. [Google Scholar]
- Webersinke, F.; Klein, H.; Flieher, M.; Urban, A.; Jäger, H.; Forster, C. Control of Fermentation By-Products and Aroma Features of Beer Produced with Scottish Ale Yeast by Variation of Fermentation Temperature and Wort Aeration Rate. J. Am. Soc. Brew. Chem. 2018, 76, 147–155. [Google Scholar] [CrossRef]
- Castro, R.; Díaz, A.B.; Durán-Guerrero, E.; Lasanta, C. Influence of different fermentation conditions on the analytical and sensory properties of craft beers: Hopping, fermentation temperature and yeast strain. J. Food Compos. Anal. 2022, 106, 104278. [Google Scholar] [CrossRef]







| Bobek | Crystal | Columbus | Polaris | |||||
|---|---|---|---|---|---|---|---|---|
| Standard | Probiotic | Standard | Probiotic | Standard | Probiotic | Standard | Probiotic | |
| °P | 3.50 ± 0.71 | 4.75 ± 0.35 | 3.00 ± 0.00 * | 5.50 ± 0.71 * | 4.75 ± 0.35 | 4.15 ± 0.21 | 2.75 ± 0.35 | 4.25 ± 0.35 |
| Alcohol (% v/v) | 4.19 ± 0.58 | 3.72 ± 0.49 | 3.98 ± 0.05 * | 3.26 ± 0.15 * | 4.90 ± 0.32 | 4.67 ± 0.57 | 4.63 ± 0.16 | 3.75 ± 0.47 |
| NTU | 52.05 ± 1.34 * | 15.50 ± 4.24 * | 40.95 ± 3.04 | 28.00 ± 3.54 | 63.40 ± 9.33 * | 16.90 ± 1.27 * | 33.10 ± 1.56 * | 13.50 ± 1.41 * |
| pH | 4.39 ± 0.03 | 4.33 ± 0.01 | 4.81 ± 0.06 * | 4.42 ± 0.04 * | 4.74 ± 0.01 * | 4.33 ± 0.09 * | 4.39 ± 0.01 * | 4.29 ± 0.01 * |
| Biterness (°IBU) | 17.95 ± 0.57 | 17.63 ± 0.18 | 14.63 ± 2.09 | 13.15 ± 0.64 | 40.73 ± 0.88 * | 46.03 ± 0.46 * | 38.23 ± 0.04 * | 37.28 ± 0.11 * |
| Color (EBC) | 17.81 ± 0.02 | 17.19 ± 0.55 | 14.14 ± 0.12 * | 12.45 ± 0.35 * | 16.24 ± 0.34 | 15.90 ± 0.25 | 15.51 ± 0.30 | 14.94 ± 0.30 |
| TPI | 23.08 ± 0.74 | 28.46 ± 1.67 | 21.56 ± 0.57 | 21.62 ± 0.48 | 22.02 ± 0.08 * | 29.72 ± 0.11 * | 23.50 ± 0.42 | 22.64 ± 0.57 |
| Compounds | Type of Yeast | Hop Variety | ||
|---|---|---|---|---|
| F-Ratio | p-Value | F-Ratio | p-Value | |
| Ethyl acetate | 5.6636 | 0.0244 * | 0.4811 | 0.6983 |
| Ethyl butyrate | 10.4308 | 0.0032 * | 0.3434 | 0.7942 |
| Ethyl isovalerate | 7.1681 | 0.0123 * | 0.5626 | 0.6445 |
| Isopentyl acetate | 7.6321 | 0.0100 * | 0.3396 | 0.7968 |
| 3-methyl-1-butanol | 6.2940 | 0.0182 * | 0.1527 | 0.9270 |
| Ethyl hexanoate | 0.7702 | 0.3876 | 1.0119 | 0.4033 |
| 2-propanone-1-hydroxy | 0.9707 | 0.3329 | 0.5147 | 0.6757 |
| Ethyl heptanoate | 10.4711 | 0.0031 * | 6.0744 | 0.0028 * |
| Acetic acid | 0.0297 | 0.8644 | 1.8376 | 0.1651 |
| Ethyl octanoate | 6.4916 | 0.0166 * | 0.5152 | 0.6755 |
| Heptanol | 18.5759 | 0.0002 * | 0.7303 | 0.5433 |
| Furfural | 0.4046 | 0.5299 | 1.8836 | 0.1571 |
| Benzaldehyde | 1.9839 | 0.1700 | 2.1051 | 0.1240 |
| Linalool | 3.4916 | 0.0722 | 9.3875 | 0.0002 * |
| Octanol | 5.7865 | 0.0230 * | 3.3051 | 0.0358 * |
| Isobutyric acid | 20.7668 | 0.0001 * | 0.0818 | 0.9693 |
| Ethyl decanoate | 10.1045 | 0.0036 * | 2.0291 | 0.1344 |
| Isovaleric acid | 0.4728 | 0.4974 | 1.4605 | 0.2483 |
| Hexanoic acid | 12.1402 | 0.0016 * | 1.4144 | 0.2610 |
| 1-decanol | 1.1710 | 0.2884 | 6.4809 | 0.0020 * |
| Phenethyl acetate | 30.3246 | 0.0000 * | 0.1266 | 0.9435 |
| Beta-damascenone | - | - | - | - |
| Ethyl dodecanoate | 11.8227 | 0.0018 * | 2.4504 | 0.0860 |
| Benzenepropanoic acid ethyl ester | 8.5046 | 0.0069 * | 1.9490 | 0.1465 |
| Phenethyl alcohol | 3.3308 | 0.0787 | 0.1240 | 0.9451 |
| Nerolidol | 19.2300 | 0.0001 * | 1.7629 | 0.1790 |
| Octanoic acid | 7.6275 | 0.0100 * | 2.1012 | 0.1245 |
| Nonanoic acid | 19.4279 | 0.0001 * | 1.3467 | 0.2809 |
| 2-methoxy-4-vinylphenol | 1.2870 | 0.2662 | 4.0242 | 0.0178 * |
| Decanoic acid | 32.8046 | 0.0000 * | 1.6548 | 0.2012 |
| Compounds | Probiotic | Standard | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Ethyl acetate * | 13.929 | 11.740 | 68.276 | 84.553 |
| Ethyl butyrate | 191.878 | 50.994 | 433.596 | 275.350 |
| Ethyl isovalerate | 45.737 | 27.852 | 206.499 | 222.667 |
| Isopentyl acetate | 360.246 | 113.297 | 1684.134 | 1790.154 |
| 3-methyl-1-butanol * | 45.341 | 13.006 | 76.379 | 44.571 |
| Ethyl hexanoate | 304.747 | 177.626 | 261.192 | 83.581 |
| 2-propanone-1-hydroxy | 429.688 | 544.125 | 762.039 | 1011.024 |
| Ethyl heptanoate | 11.346 | 3.533 | 8.036 | 1.942 |
| Acetic acid | 148.546 | 345.913 | 132.969 | 218.214 |
| Ethyl octanoate | 613.146 | 437.235 | 318.279 | 144.892 |
| Heptanol | 93.060 | 44.933 | 35.879 | 26.469 |
| Furfural * | 1.812 | 2.699 | 1.318 | 1.437 |
| Benzaldehyde | 70.237 | 69.558 | 41.813 | 38.508 |
| Linalool | 16.995 | 4.439 | 13.934 | 4.509 |
| Octanol | 10.830 | 2.468 | 18.730 | 12.044 |
| Isobutyric acid | 122.773 | 132.467 | 518.174 | 299.536 |
| Ethyl decanoate | 130.420 | 118.812 | 34.908 | 18.690 |
| Isovaleric acid * | 6.813 | 9.461 | 4.990 | 4.513 |
| Hexanoic acid * | 3.130 | 1.609 | 1.683 | 0.399 |
| 1-decanol | 5.788 | 2.762 | 6.913 | 2.908 |
| Phenethyl acetate | 92.186 | 42.099 | 274.397 | 117.149 |
| Beta-damascenone | <LOQ | - | <LOQ | - |
| Ethyl dodecanoate | 96.002 | 64.856 | 36.407 | 23.270 |
| Benzenepropanoic acid ethyl ester | <LOQ | - | 0.266 | 1.076 |
| Phenethyl alcohol * | 22.654 | 11.198 | 29.188 | 8.363 |
| Nerolidol * | 1.455 | 0.305 | 1.056 | 0.185 |
| Octanoic acid * | 5.702 | 3.784 | 3.035 | 0.761 |
| Nonanoic acid | 79.388 | 16.247 | 61.015 | 3.664 |
| 2-methoxy-4-vinylphenol * | 13.149 | 7.888 | 9.903 | 7.757 |
| Decanoic acid | 380.558 | 114.268 | 183.172 | 72.370 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, A.B.; Durán-Guerrero, E.; Valiente, S.; Castro, R.; Lasanta, C. Development and Characterization of Probiotic Beers with Saccharomyces boulardii as an Alternative to Conventional Brewer’s Yeast. Foods 2023, 12, 2912. https://doi.org/10.3390/foods12152912
Díaz AB, Durán-Guerrero E, Valiente S, Castro R, Lasanta C. Development and Characterization of Probiotic Beers with Saccharomyces boulardii as an Alternative to Conventional Brewer’s Yeast. Foods. 2023; 12(15):2912. https://doi.org/10.3390/foods12152912
Chicago/Turabian StyleDíaz, Ana Belén, Enrique Durán-Guerrero, Sergio Valiente, Remedios Castro, and Cristina Lasanta. 2023. "Development and Characterization of Probiotic Beers with Saccharomyces boulardii as an Alternative to Conventional Brewer’s Yeast" Foods 12, no. 15: 2912. https://doi.org/10.3390/foods12152912